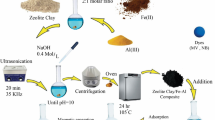
Abstract
The removal of organic dyes from the aquatic system using modified Montmorillonite clay (MMT), one of the low cost adsorbent, was studied in this paper. Polyphosphoric acid (PPA) modified MMT (PMMT) with better surface area was used for adsorbing cationic dyes such as methylene blue(MB), rhodamine B (RB) and anionic dye Rose Bengal. The adsorption capacity of the PMMT clay for MB, RB and Rose Bengal dyes were 293.9 mg g−1, 244.77 mg g−1 and 296.13 mg g−1 respectively and all follows pseudo second order kinetics with Langmuir adsorption isotherm model. For better practical application polyvinyl alchohol (PVA) thin film composite with PMMT as filler was prepared and the adsorption studies were conducted. PVA composite film with 0.5 w/v% PMMT filler was found to be a good adsorbent for the cationic dye than anionic dyes and adsorbs 99% of MB dye from 30 mgL−1 dye solution. The composite film showed pseudo second order kinetics and better fit with the Langmuir model and the mono layer adsorption on the clay surface can be explained by electrostatic interaction between adsorbent and adsorbate. The introduction of the PMMT clay in the PVA matrix increases the thermal stability of neat PVA. Tensile strength of the composite was showing a gradual increase with the amount of PMMT filler and at 0.4% addition of PMMT in PVA, the tensile strength was increased up to 39% when compared to PVA film.









Similar content being viewed by others
References
Arabahmadi V, Ghorbani M (2017) Pb (II) removal from water using surface-modified polythiophene-coated rice husk ash nanocomposite. Inorg Nano-Metal Chem 47:1614
Gupta K, Khatri OP (2017) Reduced graphene oxide as an effective adsorbent for removal of malachite green dye:plausible adsorption pathways. J Colloid Interface Sci 501:11
Sarma GK, Sengupta S, Bhattacharyya KG (2018) Adsorption of monoazo dyes ( Crocein Orange G and Procion Red MX5B ) from water using raw and acid-treated montmorillonite K10: insight into kinetics, isotherm, and thermodynamic parameters water. Air Soil Pollut 229:312
Montgomery MA, Elimelech M (2007) Water and sanitation in developing countries: Including health in the equation—Millions suffer from preventable illnesses and die every year. Environ Sci Technol 41:17
Priyanka SVC (2013) Photocatalytic oxidation of dye bearing wastewater by iron doped zinc oxide. Ind Eng Chem Res 52:17790
Mandal S, Natarajan S (2015) Adsorption and catalytic degradation of organic dyes in water using ZnO/ZnxFe3-xO4 mixed oxides. J Environ Chem Eng 3:1185
Reddy PMK, Subrahmanyam C (2012) Green approach for wastewater treatment-degradation and mineralization of aqueous organic pollutants by discharge plasma. Ind Eng Chem Res 51:11097
Elmoubarki R, Mahjoubi FZ, Tounsadi H, Moustadraf J, Abdennouri M, Zouhri A, El Albani A, Barka N (2015) Adsorption of textile dyes on raw and decanted Moroccan clays:kinetics, equilibrium and thermodynamics. Water Resour Ind 9:16
Aguiar JE, Cecilia JA, Tavares PAS, Azevedo DCS, Castellón ER, Lucena SMP, Junior IJS (2017) Adsorption study of reactive dyes onto porous clay heterostructures. Appl Clay Sci 135:35
Beall GW (2003) The use of organo-clays in water treatment. Appl Clay Sci 24:11
Hegab HM, Zou L (2015) Graphene oxide-assisted membranes: fabrication and potential applications in desalination and water purification. J Membr Sci 484:95
Robinson T, Chandran B, Nigam P (2002) Effect of pretreatments of three waste residues, wheat straw, corncobs and barley husks on dye adsorption. Bioresour Technol 85:119
Özcan AS, Özcan A (2004) Adsorption of acid dyes from aqueous solutions onto acid-activated bentonite. J Colloid Interface Sci 276:39
Gök Ö, Özcan AS, Özcan A (2010) Adsorption behavior of a textile dye of reactive blue 19 from aqueous solutions onto modified bentonite. Appl Surf Sci 256:5439
Sham AYW, Notley SM (2018) Adsorption of organic dyes from aqueous solutions using surfactant exfoliated graphene. J Environ Chem Eng 6:495
Kemp KC, Seema H, Saleh M, Le NH, Mahesh K, Chandra V, Kim KS (2013) Environmental applications using graphene composites: water remediation and gas adsorption. Nanoscale 5:3149
Chen Y, Chen L, Bai H, Li L (2013) Graphene oxide–chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J Mater Chem A 1:1992
Gadipelli S, Guo ZX (2015) Graphene-based materials: synthesis and gas sorption, storage and separation. J Prog Mater Sci 69:1
Kumar R, Suresh VM, Maji TK, Rao CNR (2014) Porous graphene frameworks pillared by organic linkers with tunable surface area and gas storage properties. Chem Comm 50:2015
Yang K, Wang J, Chen X, Zhao Q, Ghaffar A, Chen B (2018) Application of graphene-based materials in water purification: from the nanoscale to specific devices. Environ Sci Nano 5:1264
Chen G, Sun M, Wei Q, Zhang Y, Zhu B, Du B (2013) Ag 3 PO 4 /graphene-oxide composite with remarkably enhanced visible-light-driven photocatalytic activity toward dyes in water. J Hazard Mater 244–245:86
Peng N, Hu D, Zeng J, Li Y, Liang L, Chang C (2016) Superabsorbent cellulose—clay nanocomposite hydrogels for highly efficient removal of dye in water. ACS Sustain Chem Eng 4:7217
Almeida CAP, Debacher NA, Downs AJ, Cottet L, Mello CAD (2009) Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J Colloid Interface Sci 332:46
Mercier L, Detellier C (1995) Preparation, characterization, and applications as heavy metals sorbents of covalently grafted thiol functionalities on the interlamellar surface of montmorillonite. Environ Sci Technol 29:1318
Sarma GK, Sen Gupta S, Bhattacharyya KG (2016) Adsorption of crystal violet on raw and acid-treated montmorillonite, K10, in aqueous suspension. J Environ Manage 171:1
Hou P, Xing G, Han D, Wang H, Yu C, Li Y (2018) Preparation of zeolite imidazolate framework/graphene hybrid aerogels and their application as highly efficient adsorbent. J Solid State Chem 265:184
Simon-Herrero C, Gomez L, Romero A, Valverde JL, Sanchez-Silva L (2018) Nanoclay-based PVA aerogels: synthesis and characterization. Ind Eng Chem Res 57:6218
Yan J, Huang Y, Miao YE, Tjiu WW, Liu T (2015) Polydopamine-coated electrospun poly(vinyl alcohol)/poly(acrylic acid) membranes as efficient dye adsorbent with good recyclability. J Hazard Mater 283:730
Stanly S, Jelmy EJ, Nair CPR, John H (2019) Carbon dioxide adsorption studies on modified montmorillonite clay/reduced graphene oxide hybrids at low pressure. J Environ Chem Eng 7:103344
Gürses A, Doǧar Ç, Yalçin M, Açikyildiz M, Bayrak R, Karaca S (2006) The adsorption kinetics of the cationic dye, methylene blue, onto clay. J Hazard Mater 131:217
Rather LJ, Islam S, Khan MA, Mohammad F (2016) Adsorption and Kinetic studies of Adhatoda vasica natural dye onto woolen yarn with evaluations of colorimetric and fluorescence characteristics. J Environ Chem Eng 4:1780
Toor M, Jin B (2012) Adsorption characteristics, isotherm, kinetics, and diffusion of modified natural bentonite for removing diazo dy. Chem Eng J 187:79
Mouni L, Belkhiri L, Bollinger JC, Bouzaza A, Assadi A, Tirri A, Dahmoune F, Madani K, Remini H (2018) Removal of methylene blue from aqueous solutions by adsorption on Kaolin: kinetic and equilibrium studies. Appl Clay Sci 153:38
Mishra AK, Agrawal NR, Das I (2017) Synthesis of water dispersible dendritic amino acid modified polythiophenes as highly effective adsorbent for removal of methylene blue. Environ Chem Eng 5:4923
Tahir SS, Rauf N (2005) Removal of a cationic dye from aqueous solutions by adsorption onto bentonite clay. Chemosphere 63:1842
Puri C, Sumana G (2008) Highly effective adsorption of crystal violet dye from contaminated water using graphene oxide intercalated montmorillonite nanocomposite. Appl Clay Sci 166:102
Liu P, Zhang L (2007) Adsorption of dyes from aqueous solutions or suspensions with clay nano-adsorbents. Sep Purif Technol 58:32
Acisli O, Khataee A, Karaca S, Sheydaei M (2016) Modification of nanosized natural montmorillonite for ultrasound- enhanced adsorption of Acid Red 17 Ultrason. Sonochem 31:116
Unuabonah EI, Taubert A (2014) Clay-polymer nanocomposites (CPNs): adsorbents of the future for water treatment. Appl Clay Sci 99:83
Duan ZQ, Liu YT, Xie XM, Ye XY (2013) A simple and green route to transparent boron nitride/PVA nanocomposites with significantly improved mechanical and thermal properties. Chin Chem Lett 24:17
Koosha M, Mirzadeh H, Shokrgozar A, Farokhi M (2015) Nanoclay-reinforced electrospun chitosan/PVA nanocomposite nanofibers for biomedical applications. RSC Adv 5:10479
Yang JM, Su WY, Leu TL, Yang MC (2004) Evaluation of chitosan/PVA blended hydrogel membranes. J Membr Sci 236:39
Wang D, Zhu L, Qiu J, Zhu P (2020) Poly(acrylic acid)/palygorskite microgel via radical polymerization in aqueous phase for reinforcing poly(vinyl alcohol) hydrogel. Appl Clay Sci 185:105421
Dai J, Huang T, Tian SQ, Xiao YJ, Yang JH, Zhang N, Wang Y, Zhou ZW (2016) High structure stability and outstanding adsorption performance of graphene oxide aerogel supported by polyvinyl alcohol for waste water treatment. Mater Des 107:187
Acknowledgements
We gratefully acknowledge Inter University Centre for nanomaterials and Devices (IUCND), Cochin University of Science and Technology (CUSAT), Kerala, India for avail the facility for UV analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stanly, S., Jelmy, E.J. & John, H. Studies on Modified Montmorillonite Clay and Its PVA Nanohybrid for Water Purification. J Polym Environ 28, 2433–2443 (2020). https://doi.org/10.1007/s10924-020-01786-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01786-9