Abstract
In this work was described poly(d,l-lactide) microwave synthesis using tin(II) 2-ethylhexanoate initiated ring-opening polymerization. Polymerization was performed at 100 °C with monomer to initiator molar ratio ([M]/[I]) of 5,000 in 30 min. The achieved number average molar mass of obtained polymers (determined by gel permeation chromatography) was 102,320 g/mol, with the polydispersion index, Q, 2.80. Structural characterization was performed by FT-IR spectroscopy followed characteristic bands. For applicative purposes the obtained polymer was purified during the procedure of microsphere preparation. Biodegradable microspheres prepared from poly(d,l-lactide) have been widely studied in recent years and have become well established controlled drug delivery systems. In this work microspheres were loaded with allyl thiosulfinate (allicin) and its transforments products (ajoene and vinyldithiine), as pharmacological active substances. The morphology of the microspheres was analyzed using a scanning electron microscope. Allicin was synthesized by acid oxidation of allyl disufide and purification of obtained products by liquid–liquid extraction with diethyl ether. Obtained allicin, purity 73%, was transformed using microwave in acetone solution, at solvent boiling temperature, for 5 min. For the quality and quantity analysis of allicin and its transformation process was used LC/MS chromatography. (E)- and (Z)-ajoene were detected at retention time 3.1 and 3.3 min, respectively, whence 3-vynil-4H-1,2-dithiine and 2-vynil-4H-1,3-dithiine were detected at 4.3 and 4.8 min, respectively. Retention time of allicin was 2.93 min, according to liquid chromatography results. HPLC method was used for assessment of pharmaceutical substances (alicine and alicine transforments) releasing from microspheres at room temperature in solutions with different pH (pH = 3 and pH = 8) for 24 h.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polymers based on lactic acid deserve great attention due to their ability for decomposition in the human body into nontoxic metabolites. These polymers found the many applications in medicine and pharmacy, as a surgical cord [1], fracture fixer [2] or material for target therapy or controlled release of medications [3–6]. The traditional method of poly(lactide) (PLA) synthesis required rigorous conditions: high vacuum, long polymerization times, and the consumption of great quantities of energy using some metal or metal oxide as a catalyst to speed up the reaction and minimize the pyrolysis by reducing the temperature. Poly(d,l-lactide), PDLLA, is biocompatible material and can be designed to degrade within a reasonable time-scale which make this polymer useful for various biomedical and environmental applications. For controlled release system poly(d,l-lactide) can be used as matrix or microspheres carriers. Pharmaceutical substances can be released from polymer matrix, with controlled rate depending of polymer structures. The biomaterials synthesized for drug delivery must allow fine-tuning of the final properties and the adjustment of release kinetic and the degradation time. However, the release of active substances from drug is controlled by erosion of the bulk material and by the outpouring. Biodegradable microspheres prepared from PLA have been widely studied in recent years and have become well established controlled drug delivery systems [4, 6]. Poly(d,l-lactide) microspheres are mainly used for the systems with drugs which can cause undesirable effect if used in higher quantity, or in case of drugs insolubility. By the incorporation in microspheres mentioned deficiency of drugs can be diminishing, which enable safety handling for longer period of time and decreasing optimal quantity of drugs [7, 8]. Moreover, microspheres are used for local and targeted delivery, which is general requisite for drug releasing at diseased location in body [9, 10].
Allicin is a thioester of sulfonic acid, or allylthiosulfinate. It is a light yellow oily liquid with a characteristic garlic smell [11]. Pharmacologically, allicin is the most important and the most active substance and it can be obtain by extraction of fresh garlic [12–14]. Allicin isolation, determination and standardization of allicin based products are made more difficult due to the high instability and volatility. In recent decades its synthesis has become very relevant because pure allicin is hard to obtain commercially by extraction. The majority of methods of allicin synthesis refer to the oxidation of allyldisulfide by hydrogen peroxide in acid medium [15–19], oxidation of allyldisulfide with m-chlorobenzoic acid in chloroform [16], and processing of diallyldisulfide, in dichloromethane solution, by magnesium monoperoxy hydrate in the presence of ammonium-butyl sulphate [18]. Nikolic et al. [17] were investigated the mechanisms and kinetics of allicin synthesis from allyldisulfide and hydrogen peroxide in acid solution. General reaction of allicine synthesis is presented in Scheme 1.
Allicin is active against a great number of bacteria, viruses, fungi, and many other parasites [20, 21]. It inhibits the growth of Staphylococcus, Streptococcus, Bacillus, Brucella and Vibrio species in small concentrations [22] and growth of fungus Candida albicans and Aspergillus niger [23–26]. Its antimycotic effect is stronger than that of nistatin and other antimycotics [20, 27, 28]. It shows a virucidal activity against Herpes simplex type 1 and 2, Parainfluenza virus type 3, Vaccinia virus, Vesicular stomatitis virus, and Human rhinovirus type 2 [29, 30]. Beside its antimicrobial activity, allicin has an important role in clinical use in prevention of cancer and cardiovascular diseases and shows an outstanding antioxidant activity [21, 31, 32].
Degradation products of allicin in non-polar solvent, ajoene and vinyldithiine, are pharmacologicaly active substances [11, 30]. Ajoene shows strong antibacterial, antifungal and antiviral effect and is very effective in prevention of many parasite diseases [33, 34]. This, very efficient pharmacological substance, show antioxidative and antitumor effect, and also influence on hypoglycemia. Ajoene inhibits in vitro growth of bacteria Helicobacter pylori, responsible for gastro diseases as gastric ulcer or cancer [33]. Ajoene are effective in the first stadium of HIV fusion, and seems to be very promising for prevention of HIV infection [35, 36]. In the combination with chemotherapy drugs, as cytarabine and fludarabine, ajoene improved their efficiency [38, 39]. Allicin and its degradation products, ajoene and vinyldithiine, can prevent aggregation of thrombocyte, cyclooxigenase and inhibit 5-lipooxigenase. Also regulate systole and diastole blood pressure, decrease triglyceride and phospholipids content in blood [37], have diuretic properties, fibrinolitic and vasodilatoric activity [30]. Many researchers studied antimicrobial and antiviral activities of allicin, and supposed its efficacious influence against leukemia, and against growing tumor cells [36, 38, 39].
Considering the above discussion the present work involves the development of allicin loaded biodegradable microspheres of poly(d,l-lactide) for potential pharmaceutical applications. Synthesized products (microspheres, allicin and its transforments) were analyzed by GPC, FTIR, HPLC and LC/MS. Morphology of synthesized microspheres was analyzed by SEM technique. The in vitro release was performed in solution with different acidity (pH = 3 and pH = 8).
Experimental Section
Materials
d,l-Lactide (3,6-dimethyl-1,4-dioxane-2,5-dione), (98% purity) was supplied from Sigma–Aldrich Wisconsin. Tin(II) 2-ethylhexanoate (Stannous octuate), (95% purity), density 1.251 g/mL at 25 °C was from Sigma–Aldrich Wisconsin. Allyldisulfide (80% purity), density 1.008 g/mL, M = 146.8 g/mol, supplied from Aldrich Company. Hydrogen peroxide (30% purity), density 1.11 g/mL, M = 34.01 g/mol, was from Riedel-de Haën®, Germany. Chloroform, methanol and acetonitril were high-performance liquid chromatography grade. Other solvents, toluene, tetrahydrofurane (THF), acetone, diethyl ether, acetic acid and water were of reagent grade. All solvents were purchased from Merck Chemical Co. Poly(vinyl alcohol) (PVA, 88 mol% hydrolyzed, Mw 25.000) was purchased from Polysciences, Inc. (Warrington, PA, USA). Poly(styrene) standards were used to make calibration curve.
Microwave-Assisted Synthesis of Poly(d,l-lactide)
Microwave polymerization of d,l-lactide was described in detail in our previous work [40, 41]. Briefly, the dry monomer was placed in the evaporating bowls, followed by the addition of tin(II) 2-ethylhexanoate toluene solution. Polymerization was performed in a microwave reactor at 100 °C for 30 min.
Allicin Synthesis
Allicin was synthesized from allyl disulfide according to the procedure developed by Nikolic et. al [17]. Allicin was synthesised by oxidation of allyldisulfide with “acid” hydrogen peroxide (molar ratio was 1:1) at room temperature for 4 h. Reaction mixture was neutralised by sodium hydroxide solution and was separated into the two layers and allicin was obtained by liquid extraction with diethyl ether. By the evaporation of ether allicin was obtained with 73% purity [17].
Transformation of Allicin
Obtained allicin was treated by microwave in “Discover” focus microwave reactor, CEM Corporation, Matthews, NC, USA. The initial applied power was 150 W (for 5 s) after that reaction was carried out with only 5 W of power. The temperature regulation was carried out by infrared mass measuring system and maintained at 100 °C. Transformation reaction was carried out in acetone with 1:10 allicin to acetone volume ratio. Reaction was performed at 56 °C for 5 min.
Poly(D,L-Lactide) Microsphere with Allicin and Allicin Transfoments Preparation
Microspheres were prepared as follows: 50 mg of poly(d,l-lactide) granules were dissolved in 500 mL of tetrahydorfurane solution, and 5 mg of allicin and its transforments were dissolved in 25 mL of tetrahydorfurane. These solutions were added as fine fog spray to the 1 wt% of PVA water solution. The resultant mixture was then stirred at high speed at 3,000 rpm for 20 min. The solution was then centrifuged for 20 min on 4,000 rpm, decanted and dried under vacuum for a 24 h at room temperature. Dry microspheres were stored at 20 °C, in hermetically sealed containers to protect materials from air humidity.
Gel Permeation Chromatography (GPC)
The molecular masses and distribution of obtained polymers were determined by gel permeation chromatography, according to the conditions applied at our previously work [40]. For the analysis, Agilent 1100 Series system with refractive index, RID 1200, and diode array, DAD, 1200 (recording at 212 nm) detectors were used. Used column was ZORBAX PSM 300, 250 × 6.2 mm, 5 μm, covered molecular mass range 3×103–105 g/mol.
Infrared Fourier Transformation (FTIR)
Fourier transform infrared spectrum, FTIR, was recorded by Bomem Hartmann & Braun MB-series. Samples were milled with KBr (0.6 mg of the sample with 150 mg of KBr) and formed tablets under vacuum press. Recording was performed in the wave band range from 400 to 4,000 cm−1.
High Pressure Liquid Chromatography (HPLC)
Determination of allicin content in microspheres was performed by HPLC. Measurements were performed at apparatus Agilent 1100, with detector: DAD Agilent 1200, detection at 205 nm. Used column was the Zorbax Eclipse XDB-C18, 4.6 × 250 mm, operated at 25 °C. Acetonitrile/water (80/20 v/v) were used as eluent with flow 1 cm3/min. Injected volume was 20 μL.
Scanning Electron Microscopy (SEM)
The morphologies of the microspheres were observed using a scanning electron microscope (SEM, JEOL JSM—5300, Japan). The microspheres with allicin and its transforments were vacuum dried at room temperature, mounted onto brass stubs and sputter-coated with gold in an argon atmosphere using JEOL JFC—1100 ion sputter.
Liquid Chromatography-Mass Spectrometry (LC/MS)
For the analysis of transfoments products of allicin liquid chromatography-mass spectrometry, LC/MS, LCQ Fleet Ion Trap LC/MSn Thermo Scientific was used. Recording was performed using UV detector at 205 nm, in the positive ion mode, using an extractor voltage of 4.5 kV for the analysis. Used column was Zorbax Eclipse XDB-C18, 4.6 × 250 mm, operated at 25 °C. Acetonitrile/water (80/20 v/v) were used as eluent with flow 1 cm3/min. Injected volume was 20 μL.
Results and Discussion
The structures of monomer d,l-lactide, poly(d,l-lactide) and allicin are shown at Fig. 1a–c, respectively.
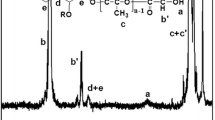
The molecular structures of the samples were confirmed by FTIR methods. Figure 2 shows characteristics bands from allicin in spectrum of loaded microspheres. It is due to some small quantity of allicin on microsphere surface. Characteristic bands of prepared samples are summarised in the Table 1.
The band at 930 cm−1 from ν(O–C=O) in lactone ring of monomer d,l-lactide, was not present in the spectra of poly(d,l-lactide) and its microspheres, which indicated the opening of lactone ring in ROP polymerization. FTIR spectrum of the poly(d,l-lactide) microspheres with alicine shows no significant differences in absorption compared to the unloaded (empty) microsphere. The absences of characteristic bands from allicin, in the spectrum of loaded microsphere, indicate embedment of allicin in polymer matrix. Band at 1,081 cm1, originated from S=O group vibration, was present in allicin FTIR spectra.
Figure 3 shows calibration curve of poly(styrene) standards, used for the determination of molecular masses and mass distribution of synthesized poly(d,l-lactide).
Figure 4 shows the signal at RID detector in function of eluation volume for sample poly(d,l-lactide) (a), and the molecular weight distribution of the the obtained poly(d,l-lactide) calculated by the software Agilent CHEMSTATION, (b). Obtained average molar masses \( \overline{\text{M}}_{\text{n}} \), \( \overline{\text{M}}_{\text{w}} \), and the polydispersivity index, Q, of the obtained poly(d,l-lactide) were: \( \overline{\text{M}}_{\text{n}} \) = 102.320 g/mol, \( \overline{\text{M}}_{\text{w}} \) = 287.110 g/mol, i Q = 2.80. The yield of poly(d,l-lactide), synthesised using microwave, was 83%.
Figure 5 shows the SEM image of poly(d,l-lactide) microspheres with allicin (a), and allicin transforments, (b), obtained by spraying a fine fog of poly(d,l-lactide) solution in tetrahydrofuran into the water solution of poly(vinyl alcohol) with intensive stirring. From SEM images it can be concluded that obtained microspheres have round shape with the sizes diameter in the ranges from 20 to 40 μm.
Synthesized allicin appears at Rt = 2.93 (retention time) in HPLC chromatograms. Mass spectra shown peak at m/z 163, originated from [MH]+ ion, form allicin. Relatively intensities and peak positions of fragmented ions were 121(90), 89 (15), 72 (3), 73 (25).
HPLC analysis of the microspheres solution (with pH = 3 and pH = 8) was used to determine allicin content in microspheres. The obtained value was 88 mg/g polymer. Alicine concentration in solution was determined by using calibration curves of methanol solution of allicin measured under the same experimental conditions (in the concentration range from 10 to 500 μg/cm3). Dependence of peak area at Rt = 2.94 min of alicine concentration was:
where P peak area (mAU*s), at Rt = 2.94 min, C allicin concentration, (mg/cm3).
Allicin concentration was calculated as dependence of peak area follow the Eq. 2:
Figure 6 shows the time dependence curves of allicin releasing during 24 h, in solution with two different pH (acid with pH = 3 and base with pH = 8).
Form the Fig. 6 it can be estimated that solution pH has strong influence the release rate of allicin. In the acid solution (belly conditions) realise rate was higher than realise in base solution (intestine conditions). At the first part of allicin realise curve, was occurred outpouring from the microsphere surface or in shallow surface layer of microsphere and in the second establishes a nearly linear dependency. This means that some amount of alicine can be released from microspheres in the stomach, in the first 4 h with rapid outpouring, and the rest amount in the intestinal system, gastro-intestinal tract, can be released slowly. Furthermore, the incorporation of allicin into the microspheres increases its stability and decrease volatilization, which make allicin handling and using much safety.
Quantitative allicin transformation, in acetone, using the microwave was much faster than other synthesis described in the literature [16], and finished after 5 min. Products were analyzed by LC/MS method. At the appropriate retention times obtained products were detected, and results are summarised in Table 2.
Table 2 shows peak areas, associated with characteristic retention times of the components, with MS data. Two dominant products were geometrical isomer of (E)-ajoene and structural isomer of 2-vynil-4H-1,3-dithiine. By the changes in peak areas during the time the (E)-ajoene and 2-vynil-4H-1,3-dithiine realizing from microspheres was followed. Results are presented at Fig. 7.
Releasing of ajoene and vynildithiine from the microsphere were much effective in acid solution. Releasing profiles of ajoene and vynildithiine were likewise allicin, clearly consist of two parts. At the first part outpouring from the microsphere surface or in shallow surface layer of microsphere were dominant (initial dosage), and in the second part a nearly continual releasing by diffusion process was established (therapeutical dosage).
Conclusions
The reduction of poly(d,l-lactide) and allicin transforments synthesis duration and energy consumption by using microwaves enables a more economical production of these two very important materials. These microwave assisted synthesis were much faster than the literature data for a conventionally heated synthesis. Allicin was synthesised by conventional oxidation of allyldisulfide with “acid” hydrogen peroxide and was used as precursor for ajoene and vynildithiine preparation in microwave field. Microwave assisted synthesized poly(d,l-lactide) was used for microspheres preparation which were loaded with allicin and its transforments. From SEM images of microspheres it was concluded that the technique provides uniform sized spheres. The size of the obtained microspheres was in the range from 20 to 40 μm. The utility and potential of microsphere drug delivery systems have been demonstrated and it has been shown that tailored delivery is possible by adjusting a conditions. Microspheres were loaded by allicin and its transforments in order to investigate possibility of microspheres used as drug carriers. Kinetics of allicin and its transforments (ajoene and vynildithiine) from the microspheres were investigated in acid (pH = 3) and base (pH = 8) solution, in order to simulate conditions in the human body. Releasing of ajoene and vynildithiine from the microsphere were much effective in acid solution. The controlled release of drugs from poly(d,l-lactide) microspheres was achieved by manipulating the physical and chemical properties of the polymer as well as those of the microsphere and acidity of solvents. From the release profiles of active components from microspheres, can be concluded that releasing consis of two periods, initial and therapeutical dosage release. Initial dosage was associated to exponential part of releasing curves, in which predominantly occurs by outpouring from the microsphere surface or in shallow surface layer of microsphere. Nearly continual releasing profiles were associated with diffusion process from microspheres (therapeutical dosage). Microspheres provide sustained release in localized areas (conditioned by pH of environment) and can be employed to reduce drug doses and its frequency of use.
References
Pennings JP, Dijkstra H, Pennings AJ (1993) Polymer 34:942
Leenslag JW, Pennings AJ, Rozema FR, Boering G (1987) Biomaterials 8:311
Anastas PT, Zimmerman JB (2003) Environ Sci Technol 37:94
Herrmann J, Bodmeier RJ (1995) Control Release 36:63
Rytting E, Nguyen J, Wang X, Kissel T (2008) Expert Opin Drug Deliv 5:629
Ravindra S, Varaprasad K, Narayana N, Vimala K, Mohana Raju K (2011) J Polym Environ 19(2):413
Davis SS, Hardy JG, Taylor MJ, Whalley DR, Willson CG (1984) Int J Pharm 21:167
Kumar ABM, Rao KP (1998) Biomaterials 19:725
Deurloo MJM, Bohlken S, Kop W, Lerk CF, Hennink W, Bartelink H, Begg AC (1990) Cancer Chemother Phamacol 27:135
Liu Z, Bendayan R, Wu XY (2001) J Pharm Pharmacol 53:779
Sticher O (1991) Dtsch Apoth Ztg 131:403
Koch J, Berger L, Reiter CV (1989) Planta Med 55:327
Lawson LD, Wood SG, Hughers BG (1991) Planta Med 57:263
Lawson LD, Wang ZJ, Hughes BG (1991) Planta Med 57:363
Freeman F, Kodera Y (1995) J Agric Food Chem 43:2332
Block E, Ahmad S, Catalfamo LJ, Jain KM, Apitz-Castrozd R (1986) J Am Chem Soc 108:7045
Nikolić V, Stanković M, Kapor A, Nikolić L, Cvetković D, Stamenković J (2004) Pharmazie 59:10
Broe ME, Paulus GJ, Verpooten GA, Roels F, Buyssens N, Wedeen RN, Van Hoof F, Tulkens PM (1984) Kidney Int 25:643
Ali BH (1995) Gen Pharmacol 26:1477
Adetumbi MA, Lau BH (1983) Med Hypothesis 12:227
Hughes BG, Lawson LD (1991) Phytother Res 5:154
Cavallito CJ, Bailey JH (1944) J Am Chem Soc 66:1950
Nikolić V, Stanković M, Nikolić L, Cvetković D, Kapor A, Cakić M (2005) Chem Ind Chem Eng Q 2:69
Ilić DP, Nikolić VD, Nikolić LB, Stanković MZ, Stanojević LP (2010) Chem Ind 64:85
Nikolić V, Stanković M, Kapor A, Nikolić L, Cvetković D, Stamenković J (2004) Pharmazie 59:845
Nikolić VD, Stanković MZ, Nikolić LB, Cvetković DM, Skala DU (2004) Chem Ind 58:109
Moore GS, Atkins RD (1977) Mycologia 69:341
Prasad G, Sharma VD (1980) Br Vet J 136:448
Nagai K (1973) Jpn J Infect Dis 47:321
Weber ND (1992) Planta Med 58:417
Taucher J, Hansel A, Jordan A, Lindinger W (1996) J Agric Food Chem 44:3778
Briggs HW, Xiao H, Parkin LK, Shen C, Goldman LI (2000) J Agric Food Chem 48:5731
Canizares P, Gracia I, Gomez LA, Martin de Argila C, Rafael L, Garcia A (2002) Biotehnol Prog 18:1227
Turos E, Revell KD, Ramaraju P, Gergeres DA, Greenhalgh K, Young A, Sathyanarayan N, Dickey S, Lim D, Alhamadsheh MM, Reynolds K (2008) Bioorg Med Chem 16:6501
Walder R, Kalvatchev Z, Aptiz-Castro R (1998) Biomed Pharmacother 52:229
Walder R, Kalvatcev Z, Garzaro D, Barrios M, Apitz-Castro R (1997) Biomed Pharmacother 51:397
Sanchez-Moreno C (2002) Int J Food Sci Technol 3:121
Li M, Min Ji M, Cui Jing R, Zhang Li H, Wang K, Valette A (2002) Nutr Cancer 42:241
Apitz-Castro R, Badimon JJ, Badimon LA (1994) Thromb Res 75:243
Nikolić L, Ristić I, Adnadjević B, Nikolić V, Jovanović J, Stanković M (2010) Sensors 10:5053
Ristić IS, Tanasić L, Nikolić L, Cakić SM, Ilić OZ, Radičević RZ, Budinski-Simendić JK (2011) J Polym Environ 19(2):419
Acknowledgments
Authors wish to express their gratitude to the Ministry of Science and Technological Development of the Republic of Serbia (Project No. TR34012) for financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ilić, D., Ristić, I.S., Nikolić, L. et al. Characterization and Release Kinetics of Allylthiosufinate and its Transforments from Poly(d,l-Lactide) Microspheres. J Polym Environ 20, 80–87 (2012). https://doi.org/10.1007/s10924-011-0337-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-011-0337-x