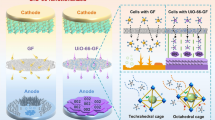
Abstract
In this work, we have theoretically investigated the adsorption of Na and Na+ ions on Ga12N12, Ga12P12, and Ga12As12 nanocages as anode materials for sodium-ion batteries (SIBs) using density functional theory (DFT). The geometrical parameters, interaction energy (Eint), frontier molecular orbitals (FMOs), and electrochemical properties of neutral and cationic Na with the nanocages were comprehensively examined. Based on the results, the structural parameter reveals that the Na atom binds strongly to N, P, and As atoms of the nanocages. Among the complexes, the smallest bond distance of 2.206 Å is noted for Na/Ga12N12 nanocage. Additionally, higher interaction energy of − 57.99 kcal/mol is observed for Na+/Ga12N12 and the FMOs analysis illustrates that Na+ has more significant interaction than the neutral one. Furthermore, encapsulating the complexes with halide (F−, Cl− and Br−) results in higher cell voltage (Vcell) on comparison with the bare nanocage of SIBs. The overall analysis illustrates that fluorine encapsulated Na/Ga12N12 has more Vcell than chlorine and bromine.









Similar content being viewed by others
References
A.J. Haider, A.A. Al-Tabbakh, A.B. Al-Zubaidi, R.A. Rsool, Preparation and characterization of LiCo0.5Ni0.45Ag0.05O2 cathode material for lithium–ion battery. J. Mater. Sci. Mater. Electron. 29, 13277–13285 (2018). https://doi.org/10.1007/s10854-018-9451-z
W. Tang, Y. Zhu, Y. Hou, L. Liu, Y. Wu, K.P. Loh, H. Zhang, K. Zhu, Aqueous rechargeable lithium batteries as an energy storage system of superfast charging. Energy Environ. Sci. 6, 2093–2104 (2013). https://doi.org/10.1039/c3ee24249h
J. Niu, Z. Zhang, D. Aurbach, Alloy anode materials for rechargeable Mg ion batteries. Adv. Energy Mater. 10, 1–33 (2020). https://doi.org/10.1002/aenm.202000697
Y. Nishi, Lithium ion secondary batteries; past 10 years and the future. J. Power Sources. 100, 101–106 (2001). https://doi.org/10.1016/S0378-7753(01)00887-4
A.A. Qayyum, Z.S. Khan, S. Ashraf, N. Ahmed, Amorphous codoped SnS/CNTs nanocomposite with improved capacity retention as an advanced sodium-ion battery anode. J. Mater. Sci. Mater. Electron. 31, 14521–14530 (2020). https://doi.org/10.1007/s10854-020-04012-3
N. Kosar, M. Asgar, K. Ayub, T. Mahmood, Halides encapsulation in aluminum/boron phosphide nanoclusters: an effective strategy for high cell voltage in Na-ion battery. Mater. Sci. Semicond. Process. 97, 71–79 (2019). https://doi.org/10.1016/j.mssp.2019.03.011
J.M. Tarascon, Key challenges in future Li-battery research. Philos. Trans. R. Soc. A 368, 3227–3241 (2010). https://doi.org/10.1098/rsta.2010.0112
R.C. Massé, E. Uchaker, G. Cao, Beyond Li-ion: electrode materials for sodium- and magnesium-ion batteries. Sci. China Mater. 58, 715–766 (2015). https://doi.org/10.1007/s40843-015-0084-8
S. Chen, Z. Ao, B. Sun, X. Xie, G. Wang, Porous carbon nanocages encapsulated with tin nanoparticles for high performance sodium-ion batteries. Energy Storage Mater. 5, 180–190 (2016). https://doi.org/10.1016/j.ensm.2016.07.001
S. Kim, D. Seo, X. Ma, G. Ceder, K. Kang, Electrode materials for rechargeable sodium-ion batteries: potential alternatives to current lithium-ion batteries. Adv. Energy Mater. (2012). https://doi.org/10.1002/aenm.201200026
L. Cao, Y. Wang, H. Hu, J. Huang, L. Kou, Z. Xu, J. Li, A N/S-codoped disordered carbon with enlarged interlayer distance derived from cirsium setosum as high-performance anode for sodium ion batteries. J. Mater. Sci. Mater. Electron. 30, 21323–21331 (2019). https://doi.org/10.1007/s10854-019-02509-0
Y. Cao, F. Pan, H. Wang, Z. Yang, J. Sun, Density functional theory calculations for the evaluation of FePS3 as a promising anode for Mg Ion Batteries. Trans. Tianjin Univ. 26, 248–255 (2020). https://doi.org/10.1007/s12209-020-00253-9
P. Panigrahi, S.B. Mishra, T. Hussain, B.R.K. Nanda, R. Ahuja, Density functional theory studies of Si2BN nanosheets as anode materials for magnesium-ion batteries. ACS Appl. Nano Mater. 3, 9055–9063 (2020). https://doi.org/10.1021/acsanm.0c01747
T. Perveen, M. Siddiq, N. Shahzad, R. Ihsan, A. Ahmad, M.I. Shahzad, Prospects in anode materials for sodium ion batteries—a review. Renew. Sustain. Energy Rev. 119, 109549 (2020). https://doi.org/10.1016/j.rser.2019.109549
D. Li, Y. Yuan, J. Liu, M. Fichtner, F. Pan, A review on current anode materials for rechargeable Mg batteries. J. Magnes. Alloys 8, 963–979 (2020). https://doi.org/10.1016/j.jma.2020.09.017
J. Kan, H. Wang, H. Zhang, J. Shi, W. Liu, D. Li, G. Dong, Y. Yang, R. Gao, Nitrogen functionalized carbon nanocages optimized as high-performance anodes for sodium ion storage. Electrochim. Acta. 304, 192–201 (2019). https://doi.org/10.1016/j.electacta.2019.03.001
Y. Zhao, Q. Fu, D. Wang, Q. Pang, Y. Gao, A. Missiul, R. Nemausat, A. Sarapulova, H. Ehrenberg, Y. Wei, G. Chen, Co9S8@carbon yolk-shell nanocages as a high performance direct conversion anode material for sodium ion batteries. Energy Storage Mater. 18, 51–58 (2019). https://doi.org/10.1016/j.ensm.2018.09.005
N. Kosar, F. Ullah, K. Ayub, U. Rashid, M. Imran, M.N. Ahmed, T. Mahmood, Theoretical investigation of halides encapsulated Na@B40 nanocages for potential applications as anodes for sodium ion batteries. Mater. Sci. Semicond. Process. 121, 105437 (2021). https://doi.org/10.1016/j.mssp.2020.105437
P. Makarios, S. Paul, P. Gopalan, A. Angamuthu, Materials science in semiconductor processing feasibility of halide ( F−, Cl− and Br−) encapsulated Be12O12 nanocages as potential anode for metal-ion batteries—a DFT-D3 approach. Mater. Sci. Semicond. Process. 147, 106719 (2022). https://doi.org/10.1016/j.mssp.2022.106719
K. Nejati, A. Hosseinian, A. Bekhradnia, E. Vessally, L. Edjlali, Journal of molecular graphics and modelling Na-ion batteries based on the inorganic BN nanocluster anodes: DFT studies. J. Mol. Graph. Model. 74, 1–7 (2017). https://doi.org/10.1016/j.jmgm.2017.03.001
M. Noei, E. Mohammadinasab, N. Ahmadaghaei, The effect of electric field on the cell voltage of inorganic AlN nanosheet based Na–ion batteries. Inorg. Chem. Commun. 91, 29–34 (2018). https://doi.org/10.1016/j.inoche.2018.03.011
M.R.S.A. Janjua, Hydrogen as an energy currency: encapsulation of inorganic Ga12N12 with alkali metals for efficient H2 adsorption as hydrogen storage materials. J. Phys. Chem. Solids. 160, 110352 (2022). https://doi.org/10.1016/j.jpcs.2021.110352
W. Yang, X. Zhang, H. Tan, D. Yang, Y. Feng, X. Rui, Y. Yu, Gallium-based anodes for alkali metal ion batteries. J. Energy Chem. 55, 557–571 (2021). https://doi.org/10.1016/j.jechem.2020.07.035
P. Lu, C. Wu, Y. Li, Z. Yu, H. Cao, S. Wang, Investigation on structural, electronic, and magnetic properties of Mn-doped Ga12N12 clusters. J. Mater. Sci. 48, 8552–8558 (2013). https://doi.org/10.1007/s10853-013-7674-1
Z. Zhao, Z. Li, Theoretical assessment of the differences in transition metal embedded fullerene-like Ga12N12 clusters. Chem. Phys. Lett. 754, 137752 (2020). https://doi.org/10.1016/j.cplett.2020.137752
E. Tahmasebi, E. Shakerzadeh, Z. Biglari, Theoretical assessment of the electro-optical features of the group III nitrides (B12N12, Al12N12 and Ga12N12) and group IV carbides (C24, Si12C12 and Ge12C12) nanoclusters encapsulated with alkali metals (Li, Na and K). Appl. Surf. Sci. 363, 197–208 (2016). https://doi.org/10.1016/j.apsusc.2015.12.001
Z. Syum, H. Woldeghebriel, The structure and electronic properties of (GaAs)n and Al/In-doped (GaAs)n (n = 2–20) clusters. Comput. Theor. Chem. 1048, 7–17 (2014). https://doi.org/10.1016/j.comptc.2014.08.026
X. Zhang, L. Jin, X. Dai, G. Chen, G. Liu, Two-dimensional GaN: an excellent electrode material providing fast ion diffusion and high storage capacity for Li-ion and Na-ion batteries. ACS Appl. Mater. Interfaces. 10, 38978–38984 (2018). https://doi.org/10.1021/acsami.8b15139
N.N. Anua, R. Ahmed, A. Shaari, B.U. Haq, M.B. Mohamad, DFT investigations of the optical properties of gallium arsenide. Adv. Mater. Res. 895, 429–438 (2014)
V. Kumar, E.V. Shah, D.R. Roy, Electronic properties of hexagonal gallium phosphide: a DFT investigation. AIP Conf. Proc. 1731, 1–4 (2016). https://doi.org/10.1063/1.4948098
C. Lee, C. Hill, N. Carolina, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Chem. Phys. Lett. 162, 165–169 (1989). https://doi.org/10.1016/0009-2614(89)85118-8
A.D. Becke, Density-functional thermochemistry. IV. A new dynamical correlation functional and implications for exact-exchange mixing. J. Chem. Phys. 104, 1040–1046 (1996). https://doi.org/10.1063/1.470829
R. Krishnan, J.S. Binkley, R. Seeger, J.A. Pople, Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 72, 650–654 (1980). https://doi.org/10.1063/1.438955
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.A. Montgomery Jr., J.E. Peralta, F. Ogliaro, M.J. Bearpark, J. Heyd, E.N. Brothers, K.N. Kudin, V.N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A.P. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N.J. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth, P. Salvador, J.J. Dannenberg, S. Dapprich, A.D. Daniels, Ö. Farkas, J.B. Foresman, J.V. Ortiz, J. Cioslowski, D.J. Fox, Gaussian 09, Revision B.01. (Gaussian, Inc, Wallingford, 2009).
K. Nejati, A. Hosseinian, A. Bekhradnia, E. Vessally, L. Edjlali, Na-ion batteries based on the inorganic BN nanocluster anodes: DFT studies. J. Mol. Graph. Model. 74, 1–7 (2017). https://doi.org/10.1016/j.jmgm.2017.03.001
P. Weichi, L. Haiyang, Z. Xuejing, Z. Wenming, S. Ebrahimi, AlN nanotubes and nanosheets as anode material for K-ion batteries: DFT studies. Phys. Lett. Sect. A 384, 126396 (2020). https://doi.org/10.1016/j.physleta.2020.126396
A. Hosseinian, S. Soleimani-amiri, S. Arshadi, E. Vessally, L. Edjlali, Boosting the adsorption performance of BN nanosheet as an anode of Na-ion batteries: DFT studies. Phys. Lett. Sect. A 381, 2010–2015 (2017). https://doi.org/10.1016/j.physleta.2017.04.022
Acknowledgements
The authors are sincerely thankful to “Bioinformatics resources and applications facility (BRAF), C-DAC, Pune” for providing the computational facilities for this work. Also, we acknowledge the workstation support from Computer Technology Centre (CTC) at KITS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duraisamy, P.D., Paul, S.P.M., Gopalan, P. et al. A DFT Study of Halogen (F−, Cl−, and Br−) Encapsulated Ga12X12 (X = N, P, and As) Nanocages for Sodium-Ion Batteries. J Inorg Organomet Polym 32, 4173–4185 (2022). https://doi.org/10.1007/s10904-022-02425-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02425-7