Abstract
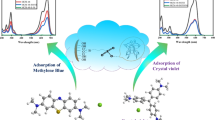
A tetrazole based metal organic framework [Cu(Metet)]n (MOF-1) (MetetH = 5-methyl-1H-tetrazole) has been prepared under solvothermal condition using sodium azide and nitrile in presence of CuCl2·2H2O. The crystalline product thus obtained was characterized by spectral (FTIR, UV–visible and fluorescence) and single crystal X-ray diffraction analysis. Tetrazole ligand is formed in situ by the reaction of azide and nitrile used. X-ray data confirm the tetrahedral geometry around the Cu(II) ion where all the four coordination sites are provided by four N of the four different ligand (Metet). MOF-1 has trigonal crystal system with R-3 m space group. Topological analysis of MOF-1 shows 4,4-c binodal net with uncommon ptr topology and stoichiometry, (4-c)(4-c). The detailed structural analysis reveals the porous nature of the MOF with channel of dimensions, Rf = 0.6 Å and Rfi = 2 Å. Further, the present MOF shows excellent adsorption properties towards organic dye, Methylene blue (MB) and thus can be employed as a good adsorbent for organic pollutants to remove MB from waste water. The possible rationale behind the dye adsorption is the porous nature of the MOF-1.
Graphic Abstract












Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
K.B. James, Macrocyclic Ligands, in Encyclopedia of Inorganic and Bioinorganic Chemistry (VCH, Weinheim 2011)
S. Park, S.Y. Lee, K.-M. Park, S.S. Lee, Acc. Chem. Res. 45, 391 (2012)
M.S. Khan, M. Khalid, M.S. Ahmad, M. Ahmad, M. Ashafaq, R. Arif, M. Shahid, J. Mol. Struct. 1175, 889 (2019)
E. Marsault, M.L. Peterson, J. Med. Chem. 54, 1961 (2011)
R. Bronisz, Inorg. Chem. 46, 6733 (2007)
X.M. Zhang, Y.F. Zhao, W.X. Zhang, X.M. Chen, Adv. Mater. 19, 2843 (2007)
M. Dinaca, J.R. Long, J. Am. Chem. Soc. 129, 11172 (2007)
H. Zhao, Z.R. Qu, H.Y. Ye, R.G. Xiong, Chem. Soc. Rev. 37, 84 (2008)
H. Deng, Y.C. Qiu, Y.H. Li, Z.H. Liu, R.H. Zeng, M. Zeller, S.R. Batten, Chem. Commun. 19, 2239–2241 (2008)
T. Wu, B.H. Yi, D. Li, Inorg. Chem. 44, 4130 (2005)
X.M. Zhang, Y.F. Zhao, H.S. Wu, S.R. Batten, S.W. Ng, Dalton Trans. 26, 3170–3178 (2006)
Z.P. Demko, K.B. Sharpless, Angew. Chem. Int. Ed. 41, 2110 (2002)
R.J. Herr, Bioorg. Med. Chem. 10, 3379 (2002)
R.E. Ford, P. Knowles, E. Lunt, S.M. Marshal, A.J. Penrose, C.A. Ramsden, A.J.H. Summers, J.L. Walker, D.E. Wrigth, J. Med. Chem. 29, 538 (1986)
E.A. Hallinan, S. Tsymbalov, C.R. Dorn, B.S. Pitzele, D.W. Hansen Jr., J. Med. Chem. 45, 1686 (2002)
J.H. Toney, P. Fitzgerald, N.G. Sharma, S.H. Olson, W.J. May, J.G. Sundelof, D.E. Vanderwall, K.A. Cleary, S.K. Grant, J.K. Wu, Chem. Biol. 5, 185 (1998)
Y. Inada, T. Wada, Y. Shibouta, M. Ojima, T. Sanada, K. Ohtsuki, K. Itoh, K. Kubo, Y. Kohara, T. Naka, J. Pharmacol. Exp. Ther. 268, 1540 (1994)
C.N.S.S.P. Kumar, D.K. Parida, A. Santhoshi, A.K. Kota, B. Sridhar, V.J. Rao, Med. Chem. Commun. 2, 486 (2011)
E. Dolusic, P. Larrieu, L. Moineaux, V. Stroobant, L. Pilotte, D. Colau, L. Pochet, B.V.D. Eynde, B. Masereel, J. Wouters, J. Med. Chem. 54, 5320 (2011)
E. Vieira, S. Huwyler, S. Jolidon, F. Knoflach, V. Mutel, J. Wichmann, Bioorg. Med. Chem. Lett. 15, 4628 (2005)
R. Huisgen, J. Sauer, H.J. Sturn, J.H. Markgraf, Chem. Ber. 93, 2106 (1960)
M. Kiskey, D.E. Chavez, D.C. Noud, S.F. Son, H.L. Berghout, C.A. Bome, Proc. Int. Pyrotech. Semin. 27, 3 (2000)
D.J. Moderhack, D.J. Moderhack, J. Prakt. Chem./Chem.-Ztg. 340, 687 (1988)
F. Himo, Z.P. Demko, L. Noodleman, K.B. Sharpless, J. Am. Chem. Soc. 125, 9983 (2003)
E.L. Chruscinska, D. Sanna, G. Micera, L. Chruscinski, J. Olejnik, R.J. Nachman, J. Zabrocki, Acta Biochim. Pol. 53, 65 (2006)
D.D. Beusen, J. Zabrocki, U. Slomczynska, R.D. Head, J.L.F. Kao, G.R. Marshall, Biopolymers 36, 181 (1995)
K.R. Knudsen, C.E.T. Mitchell, S.V. Ley, Chem. Commun. 66, 889–892 (2006)
H. Li, M. Eddaoudi, M. O’Keeffe, O.M. Yaghi, Nature 402, 276 (1999)
R.P. Ojha, P.A. Lemieux, P.K. Dixon, A.J. Liu, D.J. Durian, Nature 427, 521 (2004)
J.L.C. Rowsell, O.M. Yaghi, J. Am. Chem. Soc. 128, 1304 (2006)
R.A. Michelin, M. Mozzon, R. Bertani, Coord. Chem. Rev. 147, 299 (1996)
F. Nouar, J.F. Eubank, T. Bousquet, L. Wojtas, M.J. Zaworotko, M.J. Eddaoudi, Am. Chem. Soc. 130, 1833 (2008)
Y. Zou, S. Hong, M. Park, H. Chun, M.S. Lah, Chem. Commun. 48, 5182–5184 (2007)
J.-R. Li, R.J. Kuppler, H.-C. Zhou, Chem. Soc. Rev. 38, 1477 (2009)
K.M. Thomas, Dalton Trans. 9, 1487–1505 (2009)
Y.E. Cheon, M.P. Suh, Chem. Commun. 17, 2296–2298 (2009)
C.J. Kepert, Chem. Commun. 7, 695–700 (2006)
J. Tao, Z.J. Ma, R.B. Huang, L.S. Zheng, Inorg. Chem. 43, 6133 (2004)
F. He, M.L. Tong, X.L. Yu, X.M. Chen, Inorg. Chem. 44, 559 (2005)
G. Yang, P.P. Zhang, L.L. Liu, J.F. Kou, H.W. Hou, Y.T. Fan, CrystEngComm 11, 663 (2009)
Z. Li, M. Li, S.Z. Zhan, X.C. Huang, S.W. Ng, D. Li, CrystEngComm 10, 978 (2008)
C.F. Yan, L. Chen, R. Feng, F. Jiang, M. Hong, CrystEngComm 11, 2529 (2009)
K.C. Gordon, A.K. Burrell, T.J. Simpson, S.E. Page, G. Kelso, M.I.J. Polson, A. Flood, Eur. J. Inorg. Chem. 2002, 554 (2002)
S.S.E. Ghodsinia, B. Akhlaghinia, RSC Adv. 5, 49849 (2015)
D.M. Bowersa, A.I. Popov, Inorg. Chem. 7, 1594 (1968)
D. Wang, J. Zhang, G. Li, J. Yuan, J. Li, Q. Huo, Y. Liu, ACS Appl. Mater. Interfaces. 10, 31233 (2018)
B. Liu, Y.-C. Qiu, G. Peng, H. Deng, CrystEngComm 12, 270 (2010)
V.A. Blatov, A.P. Shevchenko, D.M. Proserpio, Cryst. Growth Des. 14, 3576 (2014)
M. O’Keeffe, M.A. Peskov, S.J. Ramsden, O.M. Yaghi, Acc. Chem. Res. 41, 1782 (2008)
E.V. Alexandrov, V.A. Blatov, A.V. Kochetkov, D.M. Proserpio, CrystEngComm 13, 3947 (2011)
J.A. Ibers, W.C. Hamilton, International Tables for X-ray Crystallography, vol. 4 (Kynoch Press, Birmingham, 1974)
SMART & SAINT Software Reference manuals, Version 6.45 (Bruker Analytical X-ray Systems, Inc., Madison, 2003)
G.M. Sheldrick, SADABS, software for empirical absorption correction, Ver. 2.05 (University of Gӧttingen, Gӧttingen, 2002)
XPREP, version 5.1 (Siemens Industrial Automation Inc., Madison, 1995)
L.J. Bourhis, O.V. Dolomanov, R.J. Gildea, J.A.K. Howard, H. Puschmann, Acta Cryst. A A71, 59 (2015)
A. Altomare, M.C. Burla, M. Camalli, G.L. Cascarano, C. Giacovazzo, A. Guagliardi, A.G.G. Moliterni, G. Polidori, R. Spagna, J. Appl. Crystallogr. 32, 115 (1999)
V.N. Serezhkin, A.V. Vologzhanina, L.B. Serezhkina, E.S. Smirnova, E.V. Grachova, P.V. Ostrova, MYu. Antipin, Acta Crystallogr. B 65, 45 (2009)
O.A. Blatova, A.A. Golov, V.A. Blatov, Zeitschrift für Kristallographie – Crystal Mater. Mat. 12, 7 (2018)
M. Raizada, F. Sama, M. Ashafaq, M. Shahid, M. Ahmad, Z.A. Siddiqi, J. Mater. Chem. C 5, 9315 (2017)
I.A. Ansari, F. Sama, M. Raizada, M. Shahid, M. Ahmad, Z.A. Siddiqi, New J. Chem. 40, 9840 (2016)
I.A. Ansari, F. Sama, M. Shahid, R. Arif, M. Khalid, Z.A. Siddiqi, RSC Adv. 6, 11088 (2016)
K. Iman, M. Shahid, M.S. Khan, M. Ahmad, F. Sama, CrystEngComm (2019). https://doi.org/10.1039/C9CE01041F
M.S. Khan, M. Khalid, M.S. Ahmad, M. Shahid, M. Ahmad, Dalton Trans. 48, 12918 (2019)
Acknowledgements
The authors thank Chairperson, Department of Chemistry, and DST-FIST and UGC DRS-SAP(II) programes for providing necessary research facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mariyam, A., Shahid, M., I., M. et al. Tetrazole Based Porous Metal Organic Framework (MOF): Topological Analysis and Dye Adsorption Properties. J Inorg Organomet Polym 30, 1935–1943 (2020). https://doi.org/10.1007/s10904-019-01334-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-019-01334-6