Abstract
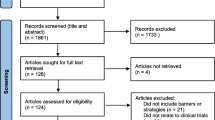
We summarize the clinical trials (CTs) main characteristics, including members of ethnic minorities from Latin America. We carried out a systematic search in six databases. We made a descriptive synthesis of CTs, summarizing the characteristics, interventions, main findings, results, and conclusions reported. 4411 studies were acquired in search strategy, leaving 24 CTs in the final selection. Of these, ten were randomized, four were non-randomized, and the remainder had other designs. Most of the studies were carried out in the population of infants and children (08), ten of the studies included only women, and two studies included men. Nine studies were conducted in Mexico, with the Mayan ethnic minority being mostly evaluated (05). In only 15 it was mentioned that their research was approved by a research ethics committee. Finally, half of the CTs reported funding from international agencies and third reported funding from government agencies. Our results show that that CTs in ethnic minorities are limited and reduced to a few native peoples of the continent.

Similar content being viewed by others
References
Instituto Nacional de Salud. Reglamento de Ensayos Clínicos [Internet]. Minist. Salud. Lima, Perú; 2018. Available from: https://repositorio.ins.gob.pe/handle/20.500.14196/1113
Asociación Española de Vacunología. Las dificultades para reclutar minorías étnicas para los ensayos clínicos. 2020. Available from: https://www.vacunas.org/las-dificultades-para-reclutar-minorias-etnicas-para-los-ensayos-clinicos/.
Food & Drugs Administration. Diversidad en los estudios clínicos: Hoja Informativa. FDA. 2022. Available from: https://www.fda.gov/consumers/minority-health-and-health-equity/diversidad-en-los-estudios-clinicos-hoja-informativa.
Vasquez EE, Perez-Brumer A, Parker RG. Social inequities and contemporary struggles for collective health in Latin America. Glob Public Health. 2019;14(6–7):777–90. https://doi.org/10.1080/17441692.2019.1601752.
Hopenhayn M, Bello Á. Discriminación étnico-racial y xenofobia en América Latina y el Caribe. Ser Polit Soc. 2001. Available from: http://orton.catie.ac.cr/cgi-bin/wxis.exe/?IsisScript=COLEC.xis&method=post&formato=2&cantidad=1&expresion=mfn=014952
Organización Panamericana de la Salud, Organización Mundial de la Salud. Política sobre etnicidad y salud. 2017;1–33.
Solano-Sandí LA, Cambronero-Valverde M, Herrera-Watson G. Identificación y análisis de los ensayos clínicos intervencionales planificados y registrados sobre COVID-19. Medwave. 2020;20(9):e8051.
Clark LT, Watkins L, Piña IL, Elmer M, Akinboboye O, Gorham M, et al. Increasing diversity in clinical trials: overcoming critical barriers. Curr Probl Cardiol Mosby. 2019;44(5):148–72.
Lolic M, Araojo R, Okeke M, Temple RUS. racial and ethnic participation in global clinical trials by therapeutic areas. J Clin Pharm Ther. 2021;46:1576–81. https://doi.org/10.1111/jcpt.13532.
Occa A, Morgan SE, Potter JNE. Underrepresentation of hispanics and other minorities in clinical trials: recruiters’ perspectives. J Racial Ethn Heal Disparities. 2018;5:322–32. https://doi.org/10.1007/s40615-017-0373-x.
Nunes JC, Rice EN, Stafford RS, Lewis EF, Wang PJ. Underrepresentation of ethnic and racial minorities in atrial fibrillation clinical trials. Circ Arrhythm Electrophysiol. 2021;14(12):e010452. https://doi.org/10.1161/CIRCEP.121.010452.
Silver JK, Flores LE, Mondriguez González A, Frontera WR. An Analysis of the inclusion of women, older individuals, and racial/ethnic minorities in rehabilitation clinical trials. Am J Phys Med Rehabil. 2021;100:546–54.
Parvanova I, Finkelstein J. Disparities in racial and ethnic representation in stem cell clinical trials. Stud Health Technol Inform. 2020;272:358–61. https://doi.org/10.3233/SHTI200569.
Regnante JM, Richie N, Fashoyin-Aje L, Hall LL, Highsmith Q, Louis JA, et al. Operational strategies in US cancer centers of excellence that support the successful accrual of racial and ethnic minorities in clinical trials. Contemp Clin Trials Commun. 2020;17:100532.
Zhang T, Tsang W, Wijeysundera HC, Ko DT. Reporting and representation of ethnic minorities in cardiovascular trials: a systematic review. Am Heart J. 2013;166:52–7.
Rosenbaum DL, Piers AD, Schumacher LM, Kase CA, Butryn ML. Racial and ethnic minority enrollment in randomized clinical trials of behavioural weight loss utilizing technology: a systematic review. Obes Rev. 2017;18(7):808–17. https://doi.org/10.1111/obr.12545.
Amorrortu RP, Arevalo M, Vernon SW, Mainous AG, Diaz V, McKee MD, et al. Recruitment of racial and ethnic minorities to clinical trials conducted within specialty clinics: an intervention mapping approach. Trials. 2018;19:1–10. https://doi.org/10.1186/s13063-018-2507-9.
Khalil L, Leary M, Rouphael N, Ofotokun I, Rebolledo PA, Wiley Z. Racial and Ethnic Diversity in SARS-CoV-2 Vaccine Clinical Trials Conducted in the United States. Vaccines. 2022;10(2):290.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13:141–6.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:1–10. https://doi.org/10.1186/s13643-016-0384-4.
Crispino V, Monárrez-Espino J. A Novel School-based Intermittent Delivery System of Iron Supplements for Highly Marginalized Tarahumara Indigenous Women of Reproductive Age of Northern Mexico. Ecol Food Nutr. 2020;59:209–25.
Carrasco Quintero MD, Ortiz Hernández L, Roldán Amaro JA, Chávez Villasana A, Aguirre Arenas J. Aguilar Carrasco FR Effect of consumption of corn flour enriched with soja on nutrition status of indigenous women of Mexico. Rev Esp Salud Publica. 2013;87:293–302.
Iannotti LL, Lutter CK, Waters WF, Riofrio CAG, Malo C, Reinhart G, et al. Eggs early in complementary feeding increase choline pathway biomarkers and DHA: a randomized controlled trial in Ecuador. Am J Clin Nutr. 2017;106:1482–9.
Lindgärde F, Ahrén B. Improved metabolic risk markers following two 6-month physical activity programs among socioeconomic marginalized women of native American ancestry in Lima. Peru Diabetes Care. 2007;30:2230–2.
Hirschler V, Maccallini G, Sanchez MS, Castaño L, Molinari C. Improvement in high-density lipoprotein cholesterol levels in argentine Indian school children after vitamin D supplementation. Horm Res Paediatr. 2013;80:335–42.
Álvarez C, Ramírez-Vélez R, Ramírez-Campillo R, Lucia A, Alonso-Martinez AM, Faúndez H, et al. Improvements cardiometabolic risk factors in Latin American Amerindians (the Mapuche) with concurrent training. Scand J Med Sci Sports. 2019;29:886–96.
Castillo-Hernandez KG, Laviada-Molina H, Hernandez-Escalante VM, Molina-Segui F, Mena-Macossay L, Caballero AE. Peer Support added to diabetes education improves metabolic control and quality of life in mayan adults living with type 2 diabetes: a randomized controlled trial. Can J Diabetes. 2021;45:206–13.
Monárrez-Espino J, López-Alarcón M, Greiner T. Randomized placebo-controlled trial of guava juice as a source of ascorbic acid to reduce iron deficiency in tarahumara indigenous schoolchildren of Northern Mexico. J Am Coll Nutr. 2011;30:191–200.
Diaz E, Bruce N, Pope D, Diaz A, Smith KR, Smith-Sivertsen T. Self-rated health among Mayan women participating in a randomised intervention trial reducing indoor air pollution in Guatemala. BMC Int Health Hum Rights. 2008;8:1–8.
Bauchet J, Undurraga EA, Zycherman A, Behrman JR, Leonard WR, Godoy RA. The effect of gender targeting of food transfers on child nutritional status: experimental evidence from the Bolivian Amazon. J Dev Eff. 2021;13:276–91.
Kao CC, Hsu JW, Dwarkanath P, Karnes JM, Baker TM, Bohren KM, et al. Indian women of childbearing age do not metabolically conserve arginine as do American and Jamaican women. J Nutr. 2015;145:884–92.
Magalhães FC, Machado-Moreira CA, Vimieiro-Gomes AC, Silami-Garcia E, Lima NRV, Rodrigues LOC, et al. Possible biphasic sweating response during short-term heat acclimation protocol for tropical natives. J Physiol Anthropol. 2006;25:215–9.
Bribiescas RG. Effects of oral Zinc supplementation on serum leptin levels in ache males of Eastern Paraguay. Am J Hum Biol. 2003;15:681–7. https://doi.org/10.1002/central/CN-00559118/full.
Lecaillon JB, Godbillon J, Guderian R, Guevara A, Cascante S, Poltera AA. Concentrations of amocarzine in plasma of 71 Ecuadorian patients of two different races receiving 3 mg/kg b.i.d. and 5 mg/kg o.d. oral postprandial doses for 3 days. Trop Med Parasitol. 1991;42:291–3. https://doi.org/10.1002/central/CN-00082585/full.
Castro-Juarez AA, Serna-Gutiérrez A, Dórame-López NA, Solano-Morales M, Gallegos-Aguilar AC, Diáz-Zavala RG, et al. effectiveness of the healthy lifestyle promotion program for yaquis with obesity and risk of diabetes in the short and medium term: a translational study. J Diabetes Res. 2020;2020:6320402.
de León ACDAC-D, González-Cortés CA, Aradillas-García C, Martínez FD-BFDB, Luevano-Contreras C. Effect of an educational intervention in capillary hemoglobin in an indigenous community in the Huasteca Potosina. Pilot study Rev Esp Nutr Humana y Diet. 2019;23:126–35.
Bevilacqua M, Morales LG, Cárdenas L, Domínguez J. Educational intervention to modify knowledge, attitudes and practices about malaria in Yékwana schools. Bol Malariol y Salud Ambient. 2015;55:155–64.
Sarmiento I, Paredes-Solís S, de Jesús García A, Maciel Paulino N, de Los Serrano, Santos FR, Legorreta-Soberanis J, et al. Safe birth in cultural safety in southern Mexico: a pragmatic non-inferiority cluster-randomised controlled trial. BMC Pregnancy Childbirth. 2022. https://doi.org/10.1186/s12884-021-04344-w.
Chomat AM, Menchú AI, Andersson N, Ramirez-Zea M, Pedersen D, Bleile A, et al. Women’s circles as a culturally safe psychosocial intervention in Guatemalan indigenous communities: a community-led pilot randomised trial. BMC Womens Health. 2019;19(1):53. https://doi.org/10.1186/s12905-019-0744-z.
Jensen ML, Frongillo EA, Leroy JL, Blake CE. Participating in a food-assisted maternal and child nutrition and health program in rural guatemala alters household dietary choices. J Nutr. 2016;146:1593–600.
Planas M-E, García PJ, Bustelo M, Carcamo CP, Martinez S, Nopo H, et al. Effects of ethnic attributes on the quality of family planning services in Lima, Peru: a randomized crossover trial. PLoS One. 2015;10:e0115274.
Rodríguez-Angulo E, Andueza-Pech G, Rosado-Alcocer L, Ortiz-Panozo E, Hernández-Prado B, Rodríguez-Ángulo E, et al. Effect of a community-based intervention to improve the knowledge on the warning signs of maternal complications among Mayan women from Yucatan randomized controlled trial. Rev Investig Clin. 2012;64:154–63.
Monarrez-Espino J, Perez-Espejo CR, Vazquez-Mendoza G, Balleza-Carreon A, Caballero-Hoyos R, Monárrez-Espino J, et al. Intervention to prevent intestinal parasitic reinfections among Tarahumara indigenous schoolchildren in northern Mexico. Rev Panam Salud Publica/Pan Am J Public Heal. 2011;30:196–203.
Magris M, Rubio-Palis Y, Alexander N, Ruiz B, Galván N, Frias D, et al. Community-randomized trial of lambdacyhalothrin-treated hammock nets for malaria control in Yanomami communities in the Amazon region of Venezuela. Trop Med Int Heal. 2007;12:392–403. https://doi.org/10.1002/central/CN-00578665/full.
Food and Drug Administration. Enhancing the Diversity of Clinical Trial Populations — Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry. 2020; Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enhancing-diversity-clinical-trial-populations-eligibility-criteria-enrollment-practices-and-trial.
U.S. Food and Drug Administration. Evaluation and Reporting of Age-, Race-, and Ethnicity-Specific Data in Medical Device Clinical Studies. Guid Ind Food Drug Adm Staff. 2017; Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/evaluation-and-reporting-age-race-and-ethnicity-specific-data-medical-device-clinical-studies
Santana RS, Leite SN. Priorities of clinical drug trials in Brazil and neglected diseases of poverty. Rev Panam Salud Publica. 2016;40:356–62.
Soto-Ordoñez S, Herrera-Añazco P, Estrada-Martínez M, Failoc-Rojas VE. Characteristics of the active clinical trials developed in the social health security of Peru. Rev del Cuerpo Med Hosp Nac Almanzor Aguinaga Asenjo. 2022;15:185–90.
Aguilera B. Trends in clinical trials performed in Chile. Rev Med Chil. 2021;149:110–8.
Traversi L, Bolaños R. Analysis of clinical drug trials in children compared to clinical drug trials in adults Argentina. Arch Argent Pediatr. 2019;117(1):34–40.
Reveiz L, Sangalang S, Glujovsky D, Pinzon CE, Asenjo Lobos C, Cortes M, et al. Characteristics of randomized trials published in latin america and the caribbean according to funding source. PLoS One. 2013;8:e56410. https://doi.org/10.1371/journal.pone.0056410.
Cucunubá ZM. Latin American scientific research prorities for COVID-19 prevention and control. Biomedica. 2020;40:9–13.
Peralta-Vera FG, Castillo-Céspedes E, Galup-Leyva M, Rucoba-Ames J, Herrera-Añazco P, Benites-Zapata VA. Factors associated with home remedy use by adults who do not attend health care facilities: evidence from peruvian population-based survey, 2019. J Health Care Poor Underserved. 2021;32:2110–24.
Herrera-Añazco P, Benites-Zapata VA, Hernández AV. 2022 Association between the non-use of health services and maltreatment based on ethnicity in Peru. J Health Care Poor Underserved. 2022;33:234–52.
del Carpio-Toia AM, Herrera-Añazco P, Benites-Zapata VA, Oliveira LE, Da Cunha Oliveira CC, Cañas DFV, et al. Ethic policy forum: inclusion of latin american ethnic minorities in clinical trials. Rev del Cuerpo Med Hosp Nac Almanzor Aguinaga Asenjo. 2022;15:422–33.
Emanuel EJ, Wendler D, Killen J, Grady C. What makes clinical research in developing countries ethical? The benchmarks of ethical research. J Infect Dis. 2004;189:930–7. https://doi.org/10.1086/381709.
Organización Panamericana de la Salud, Consejo de Organizaciones Internacionales de las Ciencias Médica. Pautas éticas internacionales para la investigación relacionada con la salud con seres humanos. Pautas éticas Int. para la Investig. biomédica en seres humanos. 2017.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by JKB-M, BC-C, DF-G and EAH-B. The first draft of the manuscript was written by PH-A and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Herrera-Añazco, P., Benites-Meza, J.K., Caira-Chuquineyra, B. et al. Ethnic Minority Participation in Clinical Trials from Latin America and the Caribbean: A Scoping Review. J Immigrant Minority Health 26, 604–622 (2024). https://doi.org/10.1007/s10903-023-01578-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10903-023-01578-y