Abstract
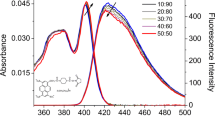
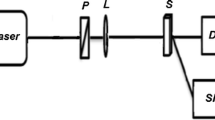
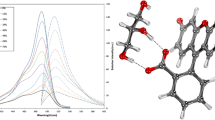
This research investigates the excimerisation of acriflavine dye in ethylene glycol and glycerol solvents. Acriflavine, a member of the acridine dye family, exhibits unique fluorescence properties with applications in various fields, including cellular nucleus observation, nucleic acid analysis, and dye laser active media etc. The study explores the impact of solvent and concentration on acriflavine's emission properties, with a focus on excimer formation, which can influence its suitability as a dye laser active medium. UV-Visible absorption spectroscopy reveals concentration-dependent absorption profiles, with distinctive monomer bands. Steady-state fluorescence studies demonstrate the emergence of red-shifted excimer fluorescence bands as concentrations increase in both solvents. Temperature-dependent fluorescence studies reveal the dynamics of excimer formation, suggesting dynamic diffusion as the excimerisation mechanism. Time-resolved fluorescence spectroscopy confirms the singlet character of both monomer and excimer states, providing insights into the excimerisation process. Critical concentration values are determined, representing the equilibrium between monomeric and excimeric forms. The study also explores pH-induced spectral shifts, highlighting the influence of acidity on fluorescence properties. Overall, this research deepens our understanding of acriflavine's excimerisation in ethylene glycol and glycerol, offering insights that can enhance its diverse applications, especially in laser technologies.










Similar content being viewed by others
Data Availability
The data generated and analysed during the present study are available from the corresponding author upon reasonable request.
Reference
Sinha TB, Singharoy D, Das HS, Gupta S, Khatua PK (2019) Photophysical studies of the dye 1-Anilinonapthalene-8-sulfonic acid in different solvents and its quantum chemical investigation. J. Mol. Struct. 1179:462–468. https://doi.org/10.1016/j.molstruc.2018.11.012
Sharma VK, Sahare PD, Rastogi RC, Ghoshal SK, Mohan D (2003) Excited state characteristics of acridine dyes: Acriflavine and acridine orange. Spectrochim Acta - Part A Mol Biomol Spectrosc 59:1799–1804. https://doi.org/10.1016/S1386-1425(02)00440-7
Lo̊ber G, (1981) The fluorescence of dye-nucleic acid complexes. J Lumin 22:221–265. https://doi.org/10.1016/0022-2313(81)90022-3
Maeda M (1984) Laser Dyes. Academic press, New York
Previtali CM (1980) CHAPTER VI Transfer Processes. In: International Geophysics. pp 261–320. https://doi.org/10.1016/S0074-6142(08)60507-0
Li Y, Kuwabara H, Gong YK et al (2003) Resonance energy transfer from dibucaine to acriflavine in polystyrene latex dispersions. J Photochem Photobiol B Biol 70:171–176. https://doi.org/10.1016/S1011-1344(03)00090-3
Chakraborty S, Hussain SA (2019) Fluorescence resonance energy transfer (FRET) between acriflavine and CdTe quantum dot. Mater Today Proc 46:6087–6090. https://doi.org/10.1016/j.matpr.2020.02.757
Misra V, Mishra H, Joshi HC, Pant TC (2000) Excitation energy transfer between acriflavine and rhodamine 6G as a pH sensor. Sensors Actuators, B Chem 63:18–23. https://doi.org/10.1016/S0925-4005(00)00296-3
Sahare PD, Sharma VK, Mohan D, Rupasov AA (2008) Energy transfer studies in binary dye solution mixtures: Acriflavine + Rhodamine 6G and Acriflavine + Rhodamine B. Spectrochim Acta - Part A Mol Biomol Spectrosc 69:1257–1264. https://doi.org/10.1016/j.saa.2007.07.003
Sorokin PP, Lankard JR (1966) Stimulated emission observed from an organic dye. IBM J Res Dev 10:162–163. https://doi.org/10.1147/rd.102.0162
Yamaguchi Y, Matsubara Y, Ochi T, Wakamiya T, Yoshida ZI (2008) How the π conjugation length affects the fluorescence emission efficiency. J Am Che Soc 130:13867–13869. https://doi.org/10.1021/ja8040493
Denisov LK, Uzhinov BM (1980) Heterocyclic compounds as the active media of lasers (review). Chem Heterocycl Compd 16:553–564. https://doi.org/10.1007/BF02400891
Latz HW (1976) Dye Lasers. In: Wehry EL (ed) Modern Fluorescence Spectroscopy. Modern Analytical Chemistry. Springer, Boston, pp 83-119
Ghanadzadeh A, Zeini A, Kashef A, Moghadam M (2009) Solvent polarizability and anisotropy effects on the photophysical behavior of oxazine 1: An appropriate polarizability indicator dye. Spectrochim Acta A Mol Biomol Spectrosc 73:324–329. https://doi.org/10.1016/j.saa.2009.02.029
Drexhage KH (1973) Structure and Properties of Laser Dyes. In: Schäfer FP (eds) Dye Lasers. Topics in Applied Physics, vol 1, Springer, Berlin, Heidelberg, pp 144–193
Zhang Y, Li D, Ou Y, Pu X, Sun Y (2019) The Study on Lasing Threshold Properties of Rhodamine B in Glycerol Aqueous Solution. IEEE Photonics J 11:1–9. https://doi.org/10.1109/JPHOT.2019.2907469
Swargiary H, Radiul SM, Kalita MPC, Hazarika S (2023) Photoexcimerisation of pure acriflavine dye in water and alcohol. J Photochem Photobiol A Chem. https://doi.org/10.1016/j.jphotochem.2023.114636
Levshin LV (1955) The effect of concentration on the optical properties of solutions of 3, 6- diaminoacridine. J Exper Theoret Phys USSR 28:201–212
Haugen GR, Melhuish WH (1964) Association and self-quenching of proflavine in water. Trans Faraday Soc 60:386–394. https://doi.org/10.1039/TF9646000386
3,6-Diamino-10-methylacridinium chloride hydrochloride (2022). https://pubchem.ncbi.nlm.nih.gov/compound/15558347#section=Spectral-Information. Accessed 12 Dec 2022
Matzke KH, Thiessen G (1976) The acridine dyes: Their purification, physicochemical, and cytochemical properties. Histochemistry 49:73–79. https://doi.org/10.1007/BF00490128
Serra F, Terentjev EM (2010) Nonlinear Absorption of Light in Materials with Long-lived Excited States. In: Evans T (ed) Nonlinear Dynamics, InTech, Europe, pp 1-30
Sato M, Azumi T, Azumi H (1967) Delayed thermal excimer fluorescence of acriflavine in a stretched PVA sheet. Bull Chem Soc Jpn 40:1031–1034
Birks JB, Dyson DJ, Munro IH (1963) Excimer fluorescence II. Lifetime Studies of pyrene solutions. Proc R Soc A: Math Phys Eng Sci 275:575–588
Stevens B, Hutton E (1960) Radiative life-time of the pyrene dimer and the possible role of excited dimers in energy transfer processes. Nature 186:1045–1046
Wen W, Hsu R (1962) Effect of dimerization upon the α-phosphorescence of acriflavine in glucose glass. J Phys Chem 66:1353–1354
Lim EC, Swenson GW (1962) Delayed fluorescence of acriflavine in rigid media. J Chem Phys 36:118–122
Fonin AV, Sulatskaya AI, Kuznetsova IM, Turoverov KK (2014) Fluorescence of dyes in solutions with high absorbance. Inner filter effect correction. PLoS One 9:e103878. https://doi.org/10.1371/journal.pone.0103878
Stevens B (1961) Evidence for the photo-association of aromatic hydrocarbons in fluid media. Nature 192:725–727
Oladepo SA (2021) Temperature-dependent fluorescence emission of 4-cyano-4′-pentylbiphenyl and 4-cyano-4′-hexylbiphenyl liquid crystals and their bulk phase transitions. J Mol Liq 323:114590
Horrocks DL (1970) Techniques for the study of excimers. In: Horrocks DL, Peng CT (eds) Proceedings of the International Conference on Organic Scintillators and Liquid Scintillation Counting, University of California, San Francisco, pp 75-90. https://doi.org/10.1016/B978-0-12-356250-0.50007-5
Picüarra S, Gomes PT, Martinho JMG (2000) Fluorescence study of the coil-globule transition of a poly(e-caprolactone) chain labeled with pyrenes at both ends. Macromolecules 33:3947–3950
Radiul SM, Chowdhury J, Goswami A, Hazarika S (2022) Fluorescence spectroscopy-based characterisation method for aggregation behaviour rhodamine B in water, ethanol, and propanol. Laser Phys 32 075602 (9pp)
Wang S, Zhang Z, Huang Z, Lei X, Wang Y, Li L, Yang L, Liu H, Sun F, Ma LJ (2021) A pyrene-based pH fluorescence probe with continuous multiple responses under acidic conditions and its application for environmental water systems and cells. J Photochem Photobiol A: Chem 418:113438. https://doi.org/10.1016/j.jphotochem.2021.113438
Radiul SM, Chowdhury J, Hazarika S (2023) Fluorescent H-aggregates of pure rhodamine B (RhB) in glycerol, ethylene glycol, methanol and butanol under ambient condition. J Mol Struct 1275:134606. https://doi.org/10.1016/j.molstruc.2022.134606
Acknowledgement
The authors thank the Science and Engineering Research Board, Department of Science and Technology, Government of India for the grant, No. CRG/2021/002942. Author SH wishes to thank FIST (DST) grant SR/FST/PSI-213/2016(C).
Funding
This work was supported by the Science and Engineering Research Board, Department of Science and Technology, Government of India (Project No. CRG/2021/002942).
Author information
Authors and Affiliations
Contributions
Simanta Hazarika: Conceptualisation, Methodology, Investigation, Supervision, Formal Analysis, Visualisation, Writing-original Draft. Hiren Swargiary: Investigation, Formal Analysis, Visualisation, Writing-original Draft. Seikh Mustafa Radiul: Investigation, Formal Analysis, Visualisation, Writing-original Draft.
Corresponding author
Ethics declarations
Ethics Approval
NA.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Swargiary, H., Radiul, S.M. & Hazarika, S. Excimer Fluorescence of Acriflavine Dye in Glycerol and Ethylene Glycol. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03686-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03686-w