Abstract
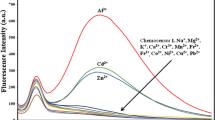
Herein, in this report we are introducing newly synthesized chalcone derivative, “(E)-1-phenyl-3-(4-((5-(((Z)-thiophen-2-ylmethylene)amino)-1,3,4-thiadiazol-2-yl)thio)phenyl)prop-2-en-1-one” (5), as a chemosensor to detect Fe2+ metal ions in HEPES buffer solution of pH 7.5. Spectroscopic techniques were used to confirm the synthesized sensor. To determine the chemical reactivity and molecular stability of the probe, a frontier molecular orbitals investigation was carried out. A molecular electrostatic potential map was investigated to know the binding site of 5 for metal ion coordination. The theoretical absorption and fluorescence emission properties were estimated and correlated with the experimental observations. The sensor showed excellent selectivity for Fe2+ compared to all other studied metal ions. The fluorescence binding studies were carried out by adding different amounts of Fe2+ ions for a fixed concentration of probe 5. The inclusion of Fe2+ ions resulted in a decrease in fluorescence intensity with a bathochromic shift of emission wavelength of 5 due to the 5-Fe2+ complexation. The binding affinity value for the probe was found to be 576.2 M−1 with the help of the Stern–Volmer plot. The Job's plot and mass spectra supported the 2:1 (5: Fe2+) stoichiometry of complex formation. The detection limit and limit of quantification of 5 for Fe2+ were calculated to be 4.79 × 10–5 M and 14.54 × 10–5 M. Further, in addition to this, the photophysical parameters such as fluorescence lifetime of 5 and 5-Fe2+ complex measured to be 0.1439 and 0.1574 ns. The quantum yield of 5 and 5-Fe2+ was found to be 0.0398 and 0.0376. All these experimental findings revealed that probe 5 has excellent selectivity and sensitivity for Fe2+ ions.












Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
Code Availability
Not applicable.
References:
Lee Jung Heon, Wang Zidong, Liu Juewen, Lu Yi (2008) Highly sensitive and selective colorimetric sensors for uranyl (UO22+): Development and comparison of labeled and label-free DNAzyme-gold nanoparticle systems. J Am Chem Soc 130:14217–14226
Luong JHT, Majid E, Male KB (2007) Analytical tools for monitoring arsenic in the environment. Open Anal Chem J 1:7–14
Korolczuk M, Moroziewicz A, Grabarczyk M (2005) Determination of subnanomolar concentrations of cobalt by adsorptive stripping voltammetry at a bismuth film electrode. Anal Bioanal Chem 382:1678–1682
Que EL, Domaille DW, Chang CJ (2008) Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem Rev 108:1517–1549
Domaille DW, Que EL, Chang CJ (2008) Synthetic fluorescent sensors for studying the cell biology of metals. Nat Chem Biol 4:168–175
Prodi L, Bolletta F, Montalti M, Zaccheroni N (2000) Luminescent chemosensors for transition metal ions. Coord Chem Rev 205:59–83
De Silva AP, Gunaratne HN, Gunnlaugsson T, Huxley AJ, McCoy CP, Rademacher JT, Rice TE (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97:1515–1566
Harsanyi G (2000). Sensors in biomedical applications: fundamentals, technology and applications (CRC press)
Mohan B, Kumar S, Sharma HK (2020) Synthesis and characterizations of flexible furfural based molecular receptor for selective recognition of Dy (III) ions. Polyhedron 183:114537
Mohan B, Ma S, Kumar S, Yang Y, Ren P (2023) Tactile sensors: Hydroxyl decorated silver metal-organic frameworks for detecting Cr2O72–, MnO4–, humic acid, and Fe3+ ions. ACS Appl Mater Interfaces 15:17317–17323
Mohan B, Kumar S, Modi K, Sharma HK, Kumar A (2021) 5-Bromo-1H-indol based flexible molecular receptor possessing spectroscopic characteristics for detection of Sm (III) and Dy (III) ions. Inorg Chim Acta 519:120275
Mason CF (2002) Biology of freshwater pollution. Pearson Education
Lopez CA, Skaar EP (2018) The impact of dietary transition metals on host-bacterial interactions. Cell Host Microbe 23:737–748
Touati D (2000) Iron and oxidative stress in bacteria. Arch Biochem Biophys 373:1–6
Beutler E (2004) “ Pumping” iron: The proteins. Science 306:2051–2053
Dai S, Schwendtmayer C, Schürmann P, Ramaswamy S, Eklund H (2000) Redox signaling in chloroplasts: cleavage of disulfides by an iron-sulfur cluster. Science 287:655–658
Atkinson A, Winge DR (2009) Metal acquisition and availability in the mitochondria. Chem Rev 109:4708–4721
Kaplan CD, Kaplan J (2009) Iron acquisition and transcriptional regulation. Chem Rev 109:4536–4552
Theil EC, Goss DJ (2009) Living with iron (and oxygen): questions and answers about iron homeostasis. Chem Rev 109:4568–4579
Abbaspour N, Hurrell R, Kelishadi R (2014) Review on iron and its importance for human health. J Res Med Sci 19:164
Camaschella C (2015) Iron-deficiency anemia. N Engl J Med 372:1832–1843
Dornelles AS, Garcia VA, de Lima MN, Vedana G, Alcalde LA, Bogo MR, Schröder N (2010) mRNA expression of proteins involved in iron homeostasis in brain regions is altered by age and by iron overloading in the neonatal period. Neurochem Res 35:564–571
Gaeta A, Hider RC (2005) The crucial role of metal ions in neurodegeneration: the basis for a promising therapeutic strategy. Br J Pharmacol 146:1041–1059
Molina-Holgado F, Hider RC, Gaeta A, Williams R, Francis P (2007) Metals ions and neurodegeneration. Biometals 20:639–654
Bhuvanesh N, Velmurugan K, Suresh S, Prakash P, John N, Murugan S, Thangadurai TD, Nandhakumar R (2017) Naphthalene based fluorescent chemosensor for Fe2+-ion detection in microbes and real water samples. J Lumin 188:217–222
Chen C-H, Cho C, Wan C-F, Wu A-T (2014) A colorimetric sensor for Fe2+ ion. Inorg Chem Commun 41:88–91
Asiri AM, Al-Ghamdi NSM, Dzudzevic-Cancar H, Kumar PKhan SA (2019) Physicochemical and Photophysical investigation of newly synthesized carbazole containing pyrazoline-benzothiazole as fluorescent chemosensor for the detection of Cu2+, Fe3+ & Fe2+ metal ion. J Mol Struct 1195:670–680
Nagarajan R, Vanjare BD, Lee KH (2021) The first tryptophan based turn-off chemosensor for Fe2+ ion detection. Spectrochim Acta Part A Mol Biomol Spectrosc 262:120103
Mohan B, Xing T, Kumar S, Kumar S, Ma S, Sun F, Xing D, Ren P (2022) A chemosensing approach for the colorimetric and spectroscopic detection of Cr3+, Cu2+, Fe3+, and Gd3+ metal ions. Sci Total Environ 845:157242
Frisch MJ, Trucks GW, Schlegel HB, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA (2013) Gaussian 09, Version D. 01; Software for Calculation. Gaussian. Inc., Wallingford, CT, USA
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. Journal of cheminformatics 4:1–17
Zhang IY, Wu J, Xu X (2010) Extending the reliability and applicability of B3LYP. Chem Commun 46:3057–3070
Jamroz MH (2004) Vibrational energy distribution analysis VEDA 4. Warsaw
Marques MA, Maitra NT, Nogueira FM, Gross EK, Rubio A (2012) Fundamentals of time-dependent density functional theory. Berlin, Heidelberg
Improta R (2011) UV–visible absorption and emission energies in condensed phase by PCM/TD‐DFT methods. Computational strategies for spectroscopy: from small molecules to nano systems. John Wiley & Sons, pp 37-75
O’boyle NM, Tenderholt AL, Langner KM (2008) Cclib: a library for package-independent computational chemistry algorithms. J Comput Chem 29:839–845
a|e - UV-Vis-IR Spectral Software 1.2, FluorTools, www.fluortools.com
Taniguchi M, Du H, Lindsey JS (2018) PhotochemCAD 3: diverse modules for photophysical calculations with multiple spectral databases. Photochem Photobiol 94:277–289
Yamuna T, Yathirajan H, Jasinski JP, Keeley AC, Narayana B, Sarojini B (2013) (2E)-1-(4-Chlorophenyl)-3-(4-nitrophenyl) prop-2-en-1-one. Acta Crystallogr Sect E: Struct Rep Online 69:o790–o791
Khamees HA, Jyothi M, Khanum SA, Madegowda M (2018) Synthesis, crystal structure, spectroscopic characterization, docking simulation and density functional studies of 1-(3, 4-dimethoxyphenyl)-3-(4-flurophenyl)-propan-1-one. J Mol Struct 1161:199–217
De Toledo T, Da Silva L, Teixeira A, Freire P, Pizani P (2015) Characterization of Meldrum’s acid derivative 5-(5-Ethyl-1, 3, 4-thiadiazol-2-ylamino) methylene-2, 2-dimethyl-1, 3-dioxane-4, 6-dione by Raman and FT-IR spectroscopy and DFT calculations. J Mol Struct 1091:37–42
Er M, Isildak G, Tahtaci H, Karakurt T (2016) Novel 2-amino-1, 3, 4-thiadiazoles and their acyl derivatives: Synthesis, structural characterization, molecular docking studies and comparison of experimental and computational results. J Mol Struct 1110:102–113
Kerru N, Gummidi L, Bhaskaruni SV, Maddila SN, Singh P, Jonnalagadda SB (2019) A comparison between observed and DFT calculations on structure of 5-(4-chlorophenyl)-2-amino-1, 3, 4-thiadiazole. Sci Rep 9:19280
Balachandran V, Janaki A, Nataraj A (2014) Theoretical investigations on molecular structure, vibrational spectra, HOMO, LUMO, NBO analysis and hyperpolarizability calculations of thiophene-2-carbohydrazide. Spectrochim Acta Part A Mol Biomol Spectrosc 118:321–330
Revanna BN, Madegowda M (2021) Dithiane based boronic acid as a carbohydrate sensor in an aqueous solution at pH 7.5: theoretical and experimental approach. J Fluoresc 31:1683–1703
Khamees HA, Revanna BN, Madegowda M, Sebastian J, Haruvegowda DB, Kumar S (2020) Structural, quantum chemical and spectroscopic investigations on photophysical properties of fluorescent saccharide sensor: Theoretical and experimental studies. ChemistrySelect 5:5227–5238
Stuart BH (2004) Infrared spectroscopy: fundamentals and applications. John Wiley & Sons
Kumar A, Kumar R, Gupta A, Tandon P, D’silva ED (2017) Molecular structure, nonlinear optical studies and spectroscopic analysis of chalcone derivative (2E)-3-[4-(methylsulfanyl) phenyl]-1-(3-bromophenyl) prop-2-en-1-one by DFT calculations. J Mol Struct 1150:166–178
Chandra S, Chowdhury J, Ghosh M, Talapatra G (2011) Adsorption of 3-thiophene carboxylic acid on silver nanocolloids: FTIR, Raman, and SERS study aided by density functional theory. J Phys Chem C 115:14309–14324
Improta R, Barone V (2004) Absorption and fluorescence spectra of uracil in the gas phase and in aqueous solution: A TD-DFT quantum mechanical study. J Am Chem Soc 126:14320–14321
Mohan B, Kumar S, Ma S, You H, Ren P (2021) Mechanistic insight into charge and energy transfers of luminescent metal–organic frameworks based sensors for toxic chemicals. Adv Sustain Syst 5:2000293
Hu S, Zhang S, Gao C, Xu C, Gao Q (2013) A new selective fluorescent sensor for Fe3+ based on a pyrazoline derivative. Spectrochim Acta Part A Mol Biomol Spectrosc 113:325–331
Khan SA (2020) Multi-step synthesis, photophysical and physicochemical investigation of novel pyrazoline a heterocyclic D-π-A chromophore as a fluorescent chemosensor for the detection of Fe3+ metal ion. J Mol Struct 1211:128084
Revanna BN, Madegowda M, Rangaswamy J, Naik N (2022) A novel Schiff base derivative as a fluorescent probe for selective detection of Cu2+ ions in buffered solution at pH 7.5: Experimental and quantum chemical calculations. J Mol Struct 1254:132327
Ingham K (1975) On the application of Job’s method of continuous variation to the stoichiometry of protein-ligand complexes. Anal Biochem 68:660–663
Shrivastava A, Gupta VB (2011) Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron Young Sci 2:21–25
Prajapati S, Sinha P, Hindore S, Jana S (2023) Selective turn-on fluorescence sensing of Fe2+ in real water samples by chalcones. Spectrochim Acta Part A Mol Biomol Spectrosc 287:122107
So H, Lee M, Kim C (2020) A unique thiosemicarbazide-based colorimetric chemosensor for Fe2+ in pure aqueous solution with the lowest detection limit. ChemistrySelect 5:10521–10525
Sasan S, Chopra T, Gupta A, Tsering D, Kapoor KK, Parkesh R (2022) Fluorescence “turn-off” and colorimetric sensor for Fe2+, Fe3+, and Cu2+ ions based on a 2, 5, 7-triarylimidazopyridine scaffold. ACS Omega 7:11114–11125
Zhu X, Duan Y, Li P, Fan H, Han T, Huang X (2019) A highly selective and instantaneously responsive Schiff base fluorescent sensor for the “turn-off” detection of iron (III), iron (II), and copper (II) ions. Anal Methods 11:642–647
Sen S, Sarkar S, Chattopadhyay B, Moirangthem A, Basu A, Dhara K, Chattopadhyay P (2012) A ratiometric fluorescent chemosensor for iron: discrimination of Fe 2+ and Fe 3+ and living cell application. Analyst 137:3335–3342
Majumdar D, Das D, Sreejith S, Das S, Biswas JK, Mondal M, Ghosh D, Bankura K, Mishra D (2019) Dicyanamide-interlaced assembly of Zn (II)-schiff-base complexes derived from salicylaldimino type compartmental ligands: Syntheses, crystal structures, FMO, ESP, TD-DFT, fluorescence lifetime, in vitro antibacterial and anti-biofilm properties. Inorg Chim Acta 489:244–254
Swamynayaka A, Srinivas MS, Vahini V, Khamees HA, Madegowda M, Hegde VN, Hegde TA, Vinitha G (2022) Third-order nonlinear optical studies of Bis (4-methylbenzylammonium) tetrachloridocuprate metal-organic crystal with optical limiting behavior: Experimental and theoretical investigations. J Mol Struct 1269:133827
Parr RG, Szentpály L, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924
Acknowledgements
The Department of Science and Technology (DST), the Government of Karnataka, and the Karnataka Science and Technology Promotion Society (K-STePS) are all acknowledged for their financial support of this study on behalf of the author, Bhavya N.R. The FT-IR, lifetime and ESI-MS data were provided by the Sophisticated Analytical Instrumentation facility (SAIF), Indian Institute of Technology, Madras, for which the authors are grateful. Additionally, University of Mysore, a University with Potential for Excellence (UPE), for providing facilities for fluorescence and UV-absorption spectroscopy. We appreciate Dr. Jeyaseelan S. for providing the computational resources.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. Methodology, Validation, Investigation, Software, Formal analysis, and writing – Original draft by Bhavya Nelligere Revanna. Conceptualization, Methodology, Supervision, writing-Review & Editing by Mahendra Madegowda. Investigations, Resources, and data collection were performed by Vinuta Kamat, Ananda Swamynayaka, Keshav Kumar Harish, Keerthikumara Venkatesha, Boja Poojary, Sanjay S. Majani and Shiva Prasad Kollur. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10895_2024_3646_MOESM1_ESM.docx
Synthesis procedure along with schemes, additional figures and tables are provided in supplementary information (SI) (DOCX 4070 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Revanna, B.N., Kamat, V., Swamynayaka, A. et al. Chalcone-based Turn-Off Chemosensor for Selective and Susceptible Detection of Fe2+ Ions: Spectroscopic and DFT Investigations. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03646-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03646-4