Abstract
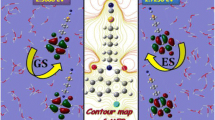
Fluorophores are powerful visualization tools and the development of novel small organic fluorophores are in great demand. Small organic fluorophores have been derived from the aurone skeleton, 2-benzylidenebenzofuran-3(2H)-one. In this study, we have utilized a model aurone derivative with a methoxy group at the 3’ position and a hydroxyl group at the 4’ position, termed vanillin aurone, to develop a foundational understanding of structural factors impacting aurone fluorescence properties. The fluorescent behaviors of the model aurone were characterized in solvent environments differing in relative polarity and dielectric constant. These data suggested that hydrogen bonding or electrostatic interactions between excited state aurone and solvent directly impact emissions properties such as peak emission wavelength, emission intensity, and Stokes shift. Time-dependent Density Functional Theory (TD-DFT) model calculations suggest that quenched aurone emissions observed in water are a consequence of stabilization of a twisted excited state conformation that disrupts conjugation. In contrast, the calculations indicate that low polarity solvents such as toluene or acetone stabilize a brightly fluorescent planar state. Based on this, additional experiments were performed to demonstrate use as a turn-on probe in an aqueous environment in response to conditions leading to planar excited state stabilization. Vanillin aurone was observed to bind to a model ATP binding protein, YME1L, leading to enhanced emissions intensities with a dissociation equilibrium constant equal to ~ 30 µM. Separately, the aurone was observed to be cell permeable with significant toxicity at doses exceeding 6.25 µM. Taken together, these results suggest that aurones may be broadly useful as turn-on probes in aqueous environments that promote either a change in relative solvent polarity or through direct stabilization of a planar excited state through macromolecular binding.











Similar content being viewed by others
Accession Codes
The YME1L construct used in this study was derived from human YME1L (Uniprot Accession Code: Q96TA2 or National Center for Biotechnology Information Genbank Accession Code: CAB51858.1).
Data Availability
All datasets and materials relevant to the current study will be made available by the authors on request. Please contact the corresponding author with questions related to access of all relevant data and materials.
Abbreviations
- ATP:
-
Adenosine triphosphate
- AAA+:
-
ATPases Associated with various cellular Activities
- YME1:
-
Yeast Mitochondrial Escape Protein 1
- NLLS:
-
Nonlinear Least Squares
- MD:
-
Molecular Dynamics
References
Boumendjel A (2003) Aurones: a subclass of flavones with promising biological potential. Curr Med Chem 10(23):2621–2630
Mazziotti I, Petrarolo G, La Motta C (2022) Aurones: a Golden Resource for active compounds. Molecules 27(1):2–21
Shanker N, Dilek O, Mukherjee K, McGee DW, Bane SL (2011) Aurones: small molecule visible range fluorescent probes suitable for biomacromolecules. J Fluoresc 21(6):2173–2184
Schmitt J, Handy ST (2019) A golden opportunity: benzofuranone modifications of aurones and their influence on optical properties, toxicity, and potential as dyes. Beilstein J Org Chem 15:1781–1785
Espinosa-Bustos C, Cortes-Arriagada D, Soto-Arriaza MA, Robinson-Duggon J, Pizarro N, Cabrera AR, Fuentealba D, Salas CO (2017) Fluorescence properties of aurone derivatives: an experimental and theoretical study with some preliminary biological applications. Photochem Photobiol Sci 16(8):1268–1276
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer
Su Y, Li K, Yu X (2019) Theoretical studies on the fluorescence enhancement of benzaldehydes by intermolecular hydrogen bonding. J Phys Chem B 123(4):884–890
Sreekanth V, Medatwal N, Kumar S, Pal S, Vamshikrishna M, Kar A, Bhargava P, Naaz A, Kumar N, Sengupta S, Bajaj A (2017) Tethering of chemotherapeutic drug/imaging agent to bile acid-phospholipid increases the efficacy and bioavailability with reduced hepatotoxicity. Bioconjug Chem 28(12):2942–2953
Kulkarni B, Surnar B, Jayakannan M (2016) Dual functional nanocarrier for cellular imaging and drug delivery in cancer cells based on pi-conjugated core and biodegradable polymer arms. Biomacromol 17(3):1004–1016
Lammers T, Kiessling F, Hennink WE, Storm G (2010) Nanotheranostics and image-guided drug delivery: current concepts and future directions. Mol Pharm 7(6):1899–1912
Kafle A, Bhattarai S, Handy ST (2020) An unusual triazole synthesis from aurones. Synthesis 52:2337–2346
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A Gen Phys 38(6):3098–3100
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785
Truong TN, Stefanovich EV (1995) A new method for incorporating solvent effect into the classical, ab initio molecular orbital and density functional theory frameworks for arbitrary shape cavity. Chem Phys Lett 240(4):253–260
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102(11):1995–2001
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-Pcm solvation model. J Comput Chem 24(6):669–681
Winget P, Dolney DM, Giesen DJ, Cramer CJ, Truhlar DG (1999) Minnesota solvent descriptor database. Institute, D. o. C. a. S. Ed. Minneapolis, MN
Bouman TD, Hansen AE, Voigt B, Rettrup S (1983) large-scale rpa calculations of chiroptical properties of organic molecules: program Rpac. Int J Quantum Chem 23(2):595–611
Cossi M, Barone V (2001) Time-dependent density functional theory for molecules in liquid solutions. J Chem Phys 115(10):4708–4717
Mewes J, You Z, Wormit M, Kriesche T, Herbert JM, Dreuw A (2015) Experimental benchmark data and systematic evaluation of two a Posteriori, polarizable-continuum corrections for vertical excitation energies in solution. J Phys Chem A 119(21):5446–5464
Cordova F, Doriol LJ, Ipatov A, Casida ME, Filippi C, Vela A (2007) Troubleshooting time-dependent density-functional theory for photochemical applications: Oxirane. J Chem Phys 127(16):164111
Hirata S, Head-Gordon M (1999) Time-dependent density functional theory within the tamm–dancoff approximation. Chem Phys Lett 314(3–4):291–299
Herbert JM (2021) Dielectric continuum methods for quantum chemistry. WIREs Comput Mol Sci 11(4):e1519
Puchades C, Rampello AJ, Shin M, Giuliano CJ, Wiseman RL, Glynn SE, Lander GC (2017) Structure of the mitochondrial inner membrane AAA + protease YME1 gives insight into substrate processing. Science 358:6363
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461
Abraham MJ, Murtola T, Schulz R, Pall S, Smith JC, Hess B, Lindahl E (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2:19–25
Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJ (2005) GROMACS: fast, flexible, and free. J Comput Chem 26(16):1701–1718
Best RB, Zhu X, Shim J, Lopes PE, Mittal J, Feig M, Mackerell AD Jr (2012) Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone phi, psi and side-chain chi(1) and chi(2) dihedral angles. J Chem Theory Comput 8(9):3257–3273
Huang J, MacKerell AD Jr (2013) CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J Comput Chem 34(25):2135–2145
Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52:7182–7190
Nosé S (1984) A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys 81:511–519
Hoover WG (1985) Canonical dynamics: equilibrium phase-space distributions. Phys Rev A 31:1695–1697
Miller JM, Brambley CA, Marsee JD (2020) Examination of the Role of Mg(2+) in the Mechanism of Nucleotide Binding to the Monomeric YME1L AAA + Domain. Biochemistry 59(45):4303–4320. https://doi.org/10.1021/acs.biochem.0c00699
Reichardt C, Welton T (2010) In solvents and solvent effects in organic chemistry, 3rd edition. Wiley Publishing. Hoboken, NJ. pp 425–508, 549–586. https://doi.org/10.1002/9783527632220
Reichardt C (1994) Solvatochromic dyes as solvent polarity indicators. Chem Rev 94(8):2319–2358
Cerón-Carrasco JP, Jacquemin D, Laurence C, Planchat A, Reichardt C, Sraïdi K (2014) Solvent polarity scales: determination of new ET(30) values for 84 organic solvents. J Phys Org Chem 27(6):512–518
Kamlet MJ, Abboud JL, Taft RW (1977) The solvatochromic comparison method. 6. The.pi.* scale of solvent polarities. J Am Chem Soc 99(18):6027–6038
Marcus Y (1998) The properties of solvents, vol 4. John Wiley & Sons Ltd, West Sussex, England
Kafle A, Bhattarai S, Miller JM, Handy ST (2020) Hydrogen sulfide sensing using an aurone-based fluorescent probe. RSC Adv 10:45180–45188
Bryant DL, Kafle A, Handy ST, Farone AL, Miller JM (2022) Aurone-derived 1,2,3-triazoles as potential fluorescence molecules in vitro. RSC Adv 12(35):22639–22649
Gozem S, Luk HL, Schapiro I, Olivucci M (2017) Theory and simulation of the ultrafast double-bond isomerization of biological chromophores. Chem Rev 117(22):13502–13565
Cardamone M, Puri NK (1992) Spectrofluorimetric assessment of the surface hydrophobicity of proteins. Biochem J 282(Pt 2) (Pt 2):589–593
Ptitsyn OB (1995) Molten globule and protein folding. Adv Protein Chem 47:83–229
Gasymov OK, Glasgow BJ (2007) ANS fluorescence: potential to augment the identification of the external binding sites of proteins. Biochim Biophys Acta 1774(3):403–411
Miller JM, Enemark EJ (2016) Fundamental characteristics of AAA + protein family structure and function. Archaea 2016:9294307
Brambley CA, Marsee JD, Halper N, Miller JM (2019) Characterization of mitochondrial YME1L protease oxidative stress-induced conformational state. J Mol Biol 431(6):1250–1266
Leonhard K, Stiegler A, Neupert W, Langer T (1999) Chaperone-like activity of the AAA domain of the yeast Yme1 AAA protease. Nature 398(6725):348–351
Weber ER, Hanekamp T, Thorsness PE (1996) Biochemical and functional analysis of the YME1 gene product, an ATP and zinc-dependent mitochondrial protease from S. Cerevisiae. Mol Biol Cell 7(2):307–317
Miller JM, Brambley CA, Marsee JD (2020) Examination of the role of mg(2+) in the mechanism of nucleotide binding to the Monomeric YME1L AAA + domain. Biochemistry 59(45):4303–4320
Erzberger JP, Berger JM (2006) Evolutionary relationships and structural mechanisms of AAA + proteins. Annu Rev Biophys Biomol Struct 35:93–114
Latt SA, Stetten G, Juergens LA, Willard HF, Scher CD (1975) Recent developments in the detection of deoxyribonucleic acid synthesis by 33258 Hoechst fluorescence. J Histochem Cytochem 23(7):493–505
Bucevicius J, Lukinavicius G, Gerasimaite R (2018) the use of hoechst dyes for DNA staining and beyond. Chemosensors 6(2):18
Han F, Taulier N, Chalikian TV (2005) Association of the minor groove binding drug Hoechst 33258 with d(CGCGAATTCGCG)2: volumetric, calorimetric, and spectroscopic characterizations. Biochemistry 44(28):9785–9794
Sandhu LC, Warters RL, Dethlefsen LA (1985) Fluorescence studies of Hoechst 33342 with supercoiled and relaxed plasmid pBR322 DNA. Cytometry 6(3):191–194
Dunn KW, Sutton TA, Sandoval RM (2018) Live-animal imaging of renal function by Multiphoton Microscopy. Curr Protoc Cytom 83:12 9 1-12 9 25
Roussaki M, Costa Lima S, Kypreou AM, Kefalas P, da Cordeiro A, Detsi A (2012) Aurones: a promising heterocyclic scaffold for the development of potent antileishmanial agents. Int J Med Chem 2012:196921
Song Z, Chen H, Fiket M, Alexander C, Chan DC (2007) OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol 178(5):749–755
Ruan Y, Li H, Zhang K, Jian F, Tang J, Song Z (2013) Loss of Yme1L perturbates mitochondrial dynamics. Cell Death Dis 4:e896
Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, Langer T (2014) The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol 204(6):919–929
Levytskyy RM, Germany EM, Khalimonchuk O (2016) Mitochondrial Quality Control Proteases in neuronal Welfare. J Neuroimmune Pharmacol 11(4):629–644
Levytskyy RM, Bohovych I, Khalimonchuk O (2017) Metalloproteases of the inner mitochondrial membrane. Biochemistry 56(36):4737–4746
Wai T, Garcia-Prieto J, Baker MJ, Merkwirth C, Benit P, Rustin P, Ruperez FJ, Barbas C, Ibanez B, Langer T (2015) Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science 350(6265):aad0116
Funding
This work was supported in part by funding to JMM/STH from the Middle Tennessee State University (MTSU) Department of Chemistry and the MTSU Molecular Biosciences (MOBI) Doctoral program. BA received financial support from the MTSU Department of Chemistry M.S. Degree program. DB received financial support from the MTSU Molecular Biosciences Doctoral program. This work used the Extreme Science and Engineering Discovery Environment (XSEDE) SDSC Expanse through allocation CHE180027.
Author information
Authors and Affiliations
Contributions
B.A., D.B., and J.M. wrote the first-draft of the manuscript and assisted in preparation of figures. B.A., D.B, S.G., and C.G. collected and analyzed experimental data for the manuscript. B.A., D.B., S.G., S.H., A.F., and J.M. collaboratively participated in the design of the study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
No ethical approvals were required to perform the work reported here (not applicable to the current study). No human or animal subjects were involved in the current study.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anderson, B., Bryant, D.L., Gozem, S. et al. Solvent-Dependent Emissions Properties of a Model Aurone Enable Use in Biological Applications. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03607-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03607-x