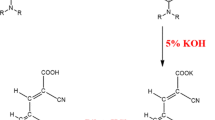
Abstract
Ruthenium-based metal complex dyes have been employed extensively in dye-sensitized solar cells (DSSCs) as photosensitizers, but the cost and toxicity of metal complexes have promoted the development of metal-free organic dyes. The present investigation deals with the synthesis of hemicyanine and Dicyanoisophorone (DCI) based dyes adopting the D-π-A strategy, and their application on sensitization of nano-crystalline ZnO electrodes by appending the carboxyl (COOH) anchoring group as a pendant on the primary skeleton of dyes. Dyes have been characterized by UV, FTIR, and NMR spectroscopic studies. Absorption maxima (λmax) were found in the region 416-551 nm while emission wavelength (λem) was observed in the range 575-685 nm. Cyclic voltammetry and DFT calculations were used to estimate redox potential and band gap energies of dyes.
Graphical Abstract


















Similar content being viewed by others
Data Availability
NA.
Code Availability (Software Application or Custom Code)
NA.
References
Gratzel M (1991) The artificial leaf, molecular photovoltaics achieve efficient generation of electricity from sunlight, Coordination. Chem Rev 111:167–174
Chen FH, Li Z (2007) Organic D-pi-A dyes for dye-sensitized solar cell. Current Org Chem 14:1241–1258
Gratzel M (2000) Perspectives for dye-sensitized nanocrystalline solar cells. Prog photovolt 8:171–185
O’Regan B, Grätzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740
Kumar R, Umar A, Kumar G, Nalwa HS, Kumar A, Akhtar MS (2017) Zinc oxide nanostructure-based dye-sensitized solar cells. J Mater Sci 52:4743–4795
Smestad GP, Gratzel M (1998) Demonstrating electron transfer and nanotechnology: a natural dye-sensitized nanocrystalline energy converter. J Chem Edu 75:752
Goncalves LM, Bermudez VD, Ribeiro HA, Mendes AM (2008) Dye-sensitized solar cells: a safe bet for the future. Energy Environmental Sci 1:655–667
Archana PS, Jose R, Vijila C, Ramakrishna S (2009) Improved electron diffusion coefficient in electrospun TiO2 nanowires. J Phys Chem C 113:21538–21542
Kaidashev EM, Lorenz MV, Von Wenckstern H, Rahm A, Semmelhack HC, Han KH, Benndorf G, Bundesmann C, Hochmuth H, Grundmann M (2003) High electron mobility of epitaxial ZnO thin films on c-plane sapphire grown by multistep pulsed-laser deposition. Appl Phys Lett 82:3901–3
Baxter JB, Walker AM, Van Ommering K, Aydil ES (2006) Synthesis and characterization of ZnO nanowires and their integration into dye-sensitized solar cells. Nanotechnol 17:S304
Mathew S, Yella A, Gao P, Humphry-Baker R, Curchod BF, Ashari-Astani N, Tavernelli I, Rothlisberger U, Nazeeruddin MK, Grätzel M (2014) Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nature chem 6:242–247
Yao Z, Zhang M, Wu H, Yang L, Li R, Wang P (2015) Donor/acceptor indenoperylene dye for highly efficient organic dye-sensitized solar cells. J Am Chem Soc 137:3799–3802
Li G, Jiang KJ, Li YF, Li SL, Yang LM (2008) Efficient structural modification of triphenylamine-based organic dyes for dye-sensitized solar cells. J Phys Chem C 112:11591–11599
Liang G, Xu J, Xu W, Wang L, Shen X, Yao M (2011) Influence of para-orientating Methoxyl Units on the Electronic Structures and Light Absorption Properties of the Triphenylamine-based dyes by DFT Study. Bull Korean Chem Soc 32:2279–2285
Xu YJ, Liang M, Liu XJ, Han HY, Sun Z, Xue S (2011) Synthesis of triarylamine dyes containing secondary electron-donating groups and application in the dye-sensitized solar cells. Synth Met 161:496–503
Liu J, Liu B, Tang Y, Zhang W, Wu W, Xie Y, Zhu WH (2015) Highly efficient cosensitization of D-A–π–A benzotriazole organic dyes with porphyrin for panchromatic dye-sensitized solar cells. J Mater Chem C 3:11144–11150
Shabir G, Saeed A, Arshad M, Zahid M (2016) "Multichromic Bis-Axially Extended Perylene Chromophore with Schiff Bases: Synthesis, Characterization and Electrochemical Studies. J fluoresce 26:2247–2255
Kay A, Cesar I, Grätzel M (2006) New benchmark for water photooxidation by nanostructured α-Fe2O3 films. J Am Chem Soc 128:15714–15721
Hara K, Sato T, Katoh R, Furube A, Ohga Y, Shinpo A, Suga S, Sayama K, Sugihara H, Arakawa H (2003) Molecular design of coumarin dyes for efficient dye-sensitized solar cells. J Phys Chem B 107:597–606
Seo KD, Song HM, Lee MJ, Pastore M, Anselmi C, De Angelis F, Nazeeruddin MK, Gräetzel M, Kim HK (2011) Coumarin dyes containing low-band-gap chromophores for dye-sensitised solar cells. Dyes Pigments 90:304–310
Saeed A, Shabir G, Arshad M (2015) Optical, Electrochemical and Thermoanalytical Investigations on Newly-Synthesized Perylene-3, 4, 9, 10-Dianhydride Fluorescent Dyes. J fluoresce 25(2015):1045–1053
Wan Z, Jia C, Zhang J, Duan Y, Lin Y, Shi Y (2012) Triphenylamine-based starburst dyes with carbazole and phenothiazine antennas for dye-sensitized solar cells. J Power Sources 199:426–431
Wan Z, Jia C, Duan Y, Zhou L, Lin Y, Shi Y (2012) Phenothiazine–triphenylamine based organic dyes containing various conjugated linkers for efficient dye-sensitized solar cells. J Mater Chem 22:25140–25147
Hua Y, Chang S, Huang D, Zhou X, Zhu X, Zhao J, Chen T, Wong WY, Wong WK (2013) Significant improvement of dye-sensitized solar cell performance using simple phenothiazine-based dyes. Chem Mater 25:2146–2153
Sudyoadsuk T, Pansay S, Morada S, Rattanawan R, Namuangruk S, Kaewin T, Jungsuttiwong S, Promarak V (2013) Synthesis and Characterization of D-D–π–A-Type Organic Dyes Bearing Carbazole-Carbazole as a Donor Moiety (D–D) for Efficient Dye-Sensitized Solar Cells. Eur J Org Chem 2013:5051–5063
Hara K, Wang ZS, Cui Y, Furube A, Koumura N (2009) Long-term stability of organic–dye-sensitized solar cells based on an alkyl-functionalized carbazole dye. Energy Environ Sci 2:1109–1114
Mehmood U, Hussein IA, Daud M, Ahmed S, Harrabi K (2015) Theoretical study of benzene/thiophene based photosensitizers for dye sensitized solar cells (DSSCs). Dyes Pigments 118:152–158
Wu Y, Marszalek M, Zakeeruddin SM, Zhang Q, Tian H, Grätzel M, Zhu W (2012) High-conversion-efficiency organic dye-sensitized solar cells: molecular engineering on D-A–π-A featured organic indoline dyes. Energy Environ Sci 5:8261–8272
Liu B, Li W, Wang B, Li X, Liu Q, Naruta Y, Zhu W (2013) Influence of different anchoring groups in indoline dyes for dye-sensitized solar cells: Electron injection, impedance and charge recombination. J Power Sources 234:139–146
Xu J, Zhang H, Liang G, Wang L, Weilin X, Cui W, Zengchang L (2010) DFT Studies on the electronic structures of indoline dyes for dye-sensitized solar cells. J Serb Chem Soc 75:259–269
Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H (2010) Dye-sensitized solar cells. Chem Rev 110:6595–6663
Manoharan S, Anandan S (2014) Cyanovinyl substituted benzimidazole based (D–π–A) organic dyes for fabrication of dye sensitized solar cells. Dyes Pigments 105:223–231
Tamilavan V, Cho N, Kim C, Ko J, Hyun MH (2012) Influences of the electron donor groups on the properties of thiophene-(N-aryl) pyrrole-thiophene-based organic sensitizers. Synth Met 162:2155–2162
Guo F, He J, Qu S, Li J, Zhang Q, Wu W, Hua J (2013) Structure-property relationship of different electron donors: new organic sensitizers based on bithiazole moiety for high efficiency dye-sensitized solar cells. RSC Adv 3:15900–15908
Shahzad N, Pugliese D, Lamberti A, Sacco A, Virga A, Gazia R, Bianco S, Shahzad MI, Tresso E, Pirri CF (2013) Monitoring the dye impregnation time of nanostructured photoanodes for dye sensitized solar cells. J Phys Conf Ser 439:012012
Sacco A, Lamberti A, Pugliese D, Chiodoni A, Shahzad N, Bianco S, Quaglio M, Gazia R, Tresso E, Pirri CF (2012) Microfluidic housing system: a useful tool for the analysis of dye-sensitized solar cell components. Appl Phys A Mater Sci Process 109:377–383
Acknowledgements
The authors gratefully acknowledge the research grant from Higher Education Commission of Pakistan under the project No. NRPU-I/10579.
Funding
This work was funded by Higher Education Commission (HEC) of Pakistan vide project number NRPU-I/10579.
Author information
Authors and Affiliations
Contributions
G. Shabir and S. Arooj has designed the work and conducted the synthesis of compounds while A. Saeed has characterized the dyes by UV and NMR studies. AH Javed, N. Shahzad and N. Iqbal conducted the application of for DSSCs. E. Jabeen Performed the DFT calculations.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
NA.
Consent for Publication
NA.
Conflict of Interest
Authors have no any conflict of interest with any other Research Group.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Research Highlights
• Design and synthesis of Fluorescent Hemicyanine and Isophorone based Photosensitizers.
• Medium to very large Stokes Shift values.
• Emission spanned over visible to NIR window I.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shabir, G., Arooj, S., Javed, A.H. et al. The Development of Highly Fluorescent Hemicyanine and Dicyanoisophorone Dyes for Applications in Dye-Sensitized Solar Cells. J Fluoresc 32, 799–815 (2022). https://doi.org/10.1007/s10895-021-02873-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02873-3