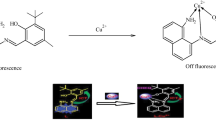
Abstract
A new acridine-based chemosensor was prepared, characterized and investigated for quantitative detection of Hg2+ ions in aqueous solutions. DFT and TD-DFT calculations showed that formation of a coordination bond between Hg2+ and the thiolate-sensor accounts for the fluorescence quenching, forming [HgLSCl2]2− as the most stable species. Limit of detection and limit of quantification were as low as 4.40 and 14.7 μmol L−1, respectively (R2 = 0.9892, least squares method), and a linear concentration range of 14.7–100 μmol L−1. Benesi-Hildebrand and Job formalisms are in accordance with the formation of a stable complex with a 1:1 (metal ion/sensor) ratio, and a determined binding constant of 5.14 × 103 L mol−1. Robustness was verified based on the variation of several analytical conditions. In addition, the method presented maximum relative standard deviation of 4.6%, and recovery results was (90.3 ± 4,6)% from distilled water, with no effect of interfering ions. Analytical figures of merit showed that the sensor can be an attractive low cost alternative for detection of Hg2+.














Similar content being viewed by others
References
Aletti AB, Gillen DM, Gunnlaugsson T (2018) Luminescent/colorimetric probes and (chemo-) sensors for detecting anions based on transition and lanthanide ion receptor/binding complexes. Coord Chem Rev 354:98–120
Kaur B, Kaur N, Kumar S (2018) Colorimetric metal ion sensors – a comprehensive review of the years 2011–2016. Coord Chem Rev 358:13–69
Chowdhurym S, Rooj B, Dutta A, Mandal U (2018) Review on recent advances in metal ions sensing using different fluorescent probes. J Fluoresc 28:999–1021
Natale FD, Lancia A, Molino A, Natale MD, Karatza D, Musmarra D (2006) Capture of mercury ions by natural and industrial materials. J Hazard Mater 132:220–225
Chatterjee S, Sarkar S, Bhattacharya S (2014) Toxic metals and autophagy. Chem Res Toxicol 27:1887–1900
Havarinasab S, Hultman P (2005) Organic mercury compounds and autoimmunity. Autoimmun Rev 4:270–275
Falter R, Schöler HF (1994) Interfacing high-performance liquid chromatography and cold-vapour atomic absorption spectrometry with on-line UV irradiation for the determination of organic mercury compounds. J Chromatogr A 675:253–256
Renzoni A, Zino F, Franchi E (1998) Mercury levels along the food chain and risk for exposed populations. Environ Res 77:68–72
Harris HH, Pickering IJ, George GN (2003) The chemical form of mercury in fish. Science 301:1203–1203
Lawson NM, Mason RP (1998) Accumulation of mercury in estuarine food chains. Biogeochemistry 40:235–247
Ynalvez R, Gutierrez J, Gonzalez-Cantu H (2016) Mini-review: toxicity of mercury as a consequence of enzyme alteration. Biometals 29:781–788
Rooney JP (2014) The retention time of inorganic mercury in the brain - a systematic review of the evidence. Toxicol Appl Pharmacol 274:425–435
Aschner M (2012) Considerations on methylmercury (MeHg) treatments in in vitro studies. Neurotoxicology 33:512–513
Zalups RK, Lash LH (2006) Cystine alters the renal and hepatic disposition of inorganic mercury and plasma thiol status. Toxicol Appl Pharmacol 214:88–97
Zahir F, Rizwi SJ, Haq SK, Khan RH (2005) Low dose mercury toxicity and human health. Environ Toxicol Pharmacol 20:351–360
Silbergeld EK, Silva IA, Nyland JF (2005) Mercury and autoimmunity: implications for occupational and environmental health. Toxicol Appl Pharmacol 207:282–292
Tchounwou PB, Ayensu WK, Ninashvili SD (2003) Environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol 18:149–175
Harada M (1995) Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol 25:1–24
Zhang C, Zhang H, Li M, Zhou Y, Zhang G, Shi L, Yao Q, Shuang S, Dong C (2019) A turn-on reactive fluorescent probe for Hg2+ in 100% aqueous solution. Talanta 197:218–224
Souza LRR, Zanatta MBT, da Silva IA, da Veiga MAMS (2018) Mercury determination in soil and sludge samples by HR CS GFAAS: comparison of sample preparation procedures and chemical modifiers. J Anal Atom Spectrom 33:1477–1485
Mo J, Li Q, Guo X, Zhang G, Wang Z (2017) Flow injection photochemical vapor generation coupled with miniaturized solution-cathode glow discharge atomic emission spectrometry for determination and speciation analysis of mercury. Anal Chem 89:10353–10360
Elias G, Marguí E, Díez S, Fontàs C (2018) Polymer inclusion membrane as an effective sorbent to facilitate mercury storage and detection by X-ray fluorescence in natural waters. Anal Chem 90:4756–4763
Shih TT, Chen JY, Luo YT, Lin CH, Liu YH, Su YA, Chao PC, Sun YC (2019) Development of a titanium dioxide-assisted preconcentration/on-site vapor-generation chip hyphenated with inductively coupled plasma-mass spectrometry for online determination of mercuric ions in urine samples. Anal Chim Acta 1063:82–90
Roshidi MDA, Fen YW, Omar NAS, Saleviter S, Daniyal WMEMM (2019) Optical studies of Graphene oxide/poly(amidoamine) Dendrimer composite thin film and its potential for sensing Hg2+ using surface Plasmon resonance SpectroscopySens. Materials 4:1147–1156
Sánchez-Calvo A, Fernández-Abedul MT, Blanco-López MC, Costa-García A (2019) Paper-based electrochemical transducer modified with nanomaterials for mercury determination in environmental waters. Sensors Actuators B Chem 290:87–92
Sivaraman G, Iniya M, Anand T, Kotla NG, Sunnapu O, Singaravadivel S, Gulyani A, Chellappa D (2018) Chemically diverse small molecule fluorescent chemosensors for copper ion Coord. Chem Rev 357:50–104
Li J, Yin C, Huo F (2016) Development of fluorescent zinc chemosensors based on various fluorophores and their applications in zinc recognition. Dyes Pigments 141:100–133
Ma LJ, Liu KL, Yin MZ, Chang J, Geng YT, Pan K (2017) Fluorescent nanofibrous membrane (FNFM) for the detection of mercuric ion (II) with high sensitivity and selectivity. Sens. Actuators B Chem 238:120–127
Zhang C, Gao B, Zhang Q, Zhang G, Shuang S, Dong C (2016) A simple Schiff base fluorescence probe for highly sensitive and selective detection of Hg2+ and Cu2+. Talanta 154:278–283
Zhang JF, Kim JS (2009) Small-molecule fluorescent chemosensors for Hg2+ ion. Anal Sci 25:1271–1281
Jiang J, Duan Q, Zheng G, Yang L, Zhang J, Wang Y, Zhang H, He J, Sun H, Ho D (2019) An ultra-sensitive and ratiometric fluorescent probe based on the DTBET process for Hg2+ detection and imaging applications. Analyst 144:1353–1360
Hazra S, Bodhak C, Chowdhury S, Sanyal D, Mandal S, Chatoopadhyay K (2019) A novel tryptamine-appended rhodamine-based chemosensor for selective detection of Hg2+ present in aqueous medium and its biological applications. Anal Bioanal Chem 411:1143–1157
Yang G, Meng X, Fang S, Duan H, Wang L, Wang Z (2019) A highly selective colorimetric fluorescent probe for detection of Hg2+ and its application on test strips. RSC Adv 9:8529–8534
Song F, Yang C, Shao X, Du L, Zhu J, Kan C (2019) A reversible “turn-off-on” fluorescent probe for real-time visualization of mercury(II) in environmental samples and its biological applications. Dyes Pigments 165:444–450
Wang Q, Jin L, Wang W, Hu T, Chen C (2019) Rhodamine derivatives as selective“naked-eye” colorimetric and fluorescence off-on sensor for Hg2+ in aqueous solution and its applications in bioimaging. J Lumin 209:411–419
Rao PG, Saritha B, Rao TS (2019) Colorimetric and turn-on fluorescence Chemosensor for Hg2+ ion detection in aqueous media. J Fluoresc 29:353–360
Elmorsi TM, Aysha TS, Sheier MB, Bedair AH (2017) Synthesis, Kinetics, and Equilibrium Study of Highly Sensitive Colorimetric Chemosensor for Monitoring of Copper Ions based on Benzo[f]fluorescein Dye Derivatives. Z Anorg Allg Che 643:811–818
Chen GQ, Guo Z, Zeng GM, Tang L (2015) Fluorescent and colorimetric sensors for environmental mercury detection. Analyst 140:5400–5443
Bera K, Das AK, Nag M, Basak S (2014) Development of a rhodamine-rhodanine-based fluorescent mercury sensor and its use to monitor real-time uptake and distribution of inorganic mercury in live zebrafish larvae. Anal Chem 86:2740–2746
Song FL, Watanabe S, Floreancig PE, Koide K (2008) Oxidation-resistant fluorogenic probe for mercury based on alkyne oxymercuration. J Am Chem Soc 130:16460–16461
Bettazzi F, Voccia D, Bencini A, Giorgi C, Palchetti I, Valtancoli B, Conti L (2018) Optical and electrochemical study of Acridine-based Polyaza ligands for anion sensing. Eur J Inorg Chem 2675-2679
Wang C, Fu J, Yao K, Chang Y, Yang L, Xu K (2018) Development of Acridine-Derived “Turn On” Al3+ Fluorescent Sensors and Their Imaging in Living Cells. Chemistry Select 3:2805–2811
Hess FK, Stewart PB (1975) Preparation of a new immunosuppressant, 4,5-bis(aminomethyl)acridine. J Med Chem 18:320–321
Laronze-Cochard M, Young-min K, Bertrand B, Riou JF, Laronze JY, Sapi J (2009) Synthesis and biological evaluation of novel 4,5-bis(dialkylaminoalkyl)-substituted acridines as potent telomeric G-quadruplex ligands. Eur J Med Chem 44:3880–3888
Maity D, Mukherjee A, Mandal SK, Roy P (2019) Modulation of fluorescence sensing properties of quinoline-based chemosensor for Zn2+: Application in cell imaging studies. J Lumin 210:508–518
Carlos FS, Nunes MC, De Boni L, Machado GS, Nunes FS (2017) A novel fluorene-derivative Schiff-base fluorescent sensor for copper(II) in organic media. J Photochem Photobiol A Chem 348:41–46
Chiron J, Galy JP (2004) Reactivity of the Acridine ring: a review. Synthesis 3:313–325
Zhang Z, Kodumuru V, Sviridov S, Liu S, Chafeev M, Chowdhury S, Cjakka N, Sun J, Gauthuer SJ, Ratkay LG, Kwan R, Thompson J, Cutts AB, Fu J, Kamboj R, Goldberg YP, Cadieux JA (2012) Discovery of benzylisothioureas as potent divalent metal transporter 1 (DMT1) inhibitors. Bioorg Med Chem 1:5108–5113
Hrdlovic P, Donovalova J, Stankovicova H, Gaplovsky A (2010) Influence of polarity of solvents on the spectral properties of bichromophoric coumarins. Molecules 15:8915–8932
Nunes MC, Carlos FS, Fuganti O, Galindo DDM, De Boni L, Abate G, Nunes FS (2020) Turn-on fluorescence study of a highly selective acridine-based chemosensor for Zn2+ in aqueous solutions. Inorg Chim Acta 499:119191. https://doi.org/10.1016/j.ica.2019.119191
G. 03, Gaussian 03, in: M.J.E.A. Frisch (Ed.) (2004) Gaussian, Inc., Wallingford CT
Schaftenaar G, Noordik JH (2000) Molden: a pre- and post-processing program for molecular and electronic structures. J Computer-Aided Mol Design 14:123–134
Schaftenaar G, Vlieg E, Vriend G (2017) Molden 2.0: quantum chemistry meets proteins. J Computer-Aided Mol Design 31:789–800
Jmol: an open-source Java viewer for chemical structures in 3D
O'Boyle NM, Tenderholt AL, Langner KM (2008) cclib: a library for package-independent computational chemistry algorithms. J Comput Chem 29:839–845
Weller M, Overton T, Rourke J, Armstrong F (2014) Inorganic chemistry, 6th edn. Oxford Univ Press, Oxford
Fabbrizzi L, Poggi A (1995) Sensors and switches from supramolecular chemistry. Chem Soc Rev 24:197–202
Espada-Bellido E, Galindo-Riaño MD, García-Vargas M, Narayanaswamy R (2010) Selective chemosensor for copper ions based on fluorescence quenching of a Schiff-base fluorophore. Appl Spectrosc 64:727–732
Roundhil DM (1994) Photochemistry and Photophysics of metal complexes. Plenum Press, New York
Hibbert DB, Gooding JJ (2006) Data analysis for chemistry - an introductory guide for students and laboratory scientists. Oxford University Press, Oxford
Miller JN, Miller JC (2005) Statistics and Chemometrics for Analytical Chemistry. 5th Ed. Edinburgh. Pearson - Prentice Hall
Ellison SLR, Barwick VJ, Farrant TJD (2009) Practical statistics for the analytical scientist- a bench guide, 2nd edn. Royal Society of Chemistry, Cambridge
Rutledge DN, Barros AS (2002) Durbin-Watson statistic as a morphological estimator of information content. Anal Chim Acta 454:277–295
Karageorgou E, Sanaribidou V (2014) Youden test application in robustness assays during method validation. J Chromatogr A 1353:131–139
Bhosale TR, Chandam DR, Anbhule PV, Deshmukh MB (2019) Synthesis of novel 4-((substituted bis-indolyl)methyl)-benzo-15-crown-5 for the colorimetric detection of Hg2+ ions in an aqueous medium. J Heterocyclic Chem 56:477–484
Huang HJ, Chir JL, Cheng HJ, Chen SJ, Hu CH, Wu AT (2011) Synthesis of highly selective Indole-based sensors for mercuric ion. J Fluoresc 21:1021–1026
Xu K, Li Y, Si Y, He Y, Ma J, He J, Hou H, Li K A “turn-on” fluorescent chemosensor for the detection of Hg(II) in buffer-free aqueous solution with excellent selectivity. J Lumin 204:182–188
Kang H, Xu H, Fan C, Liu G, Pu S (2018) A new sensitive symmetric fluorescein-linked diarylethene chemosensor for Hg2+ detection. J Photochem Photobiol A Chem 367:465–470
Zhang X, Wang Y, Yuan H, Guo X, Dai B, Jia X (2019) An acid-fluorescence and alkali-colorimetric dual channels sensor for Hg2+ selective detection by different coordination manners in aqueous media. J Photochem Photobiol A Chem 373:12–19
Joshi S, Kumari S, Sarmah A, Pant DD, Sakhuja R (2017) Detection of Hg2+ ions in aqueous medium using an indole-based fluorescent probe: experimental and theoretical investigations. J Mol Liq 248:668–677
Rice KM, Walker EM, Wu M, Gillette C, Blough ER (2014) Environmental mercury and its toxic effects. J Prev Med Public Health 47:74–83
Acknowledgements
Brazilian Research Council CNPq supported this work. Authors thank CNPq and CAPES for research fellowships. CNPq Grant # 401119/2016-5. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001”. We gratefully thank Professor Leni Campos Akcelrud (Paulo Scarpa Laboratory, UFPR)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 14760 kb)
Rights and permissions
About this article
Cite this article
Nunes, M.C., dos Santos Carlos, F., Fuganti, O. et al. A Facile Preparation of a New Water-Soluble Acridine Derivative and Application as a Turn-off Fluorescence Chemosensor for Selective Detection of Hg2+. J Fluoresc 30, 235–247 (2020). https://doi.org/10.1007/s10895-020-02489-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-020-02489-z