Abstract
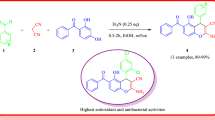
Furopyridine III, namely 1-(3-amino-4-(4-(tert-butyl)phenyl)-6-(p-tolyl)furo[2,3-b]pyridin-2-yl)ethan-1-one, synthesized from 4-(4-(tert-butyl)phenyl)-2-oxo-6-(p-tolyl)-1,2-dihydropyridine-3-carbonitrile I in two steps. The title compound is characterized by NMR, MS and its X-ray structure. The molecular structure consists of planar furopyridine ring with both phenyl rings being inclined from the furopyridine scaffold to a significant different extent. There are three intramolecular hydrogen bonds within the structure. The lattice is stabilized by N—H…O, H2C—H …π and π…π intermolecular interactions leading to three-dimensional network. Compound III exhibits fluorescent properties, which are investigated. Antimicrobial potential and antioxidant activity screening studies for the title compound III and the heterocyclic derivatives, I and II, show no activity towards neither bacterial nor fungal strains, while they exhibited weak to moderate antioxidant activity compared to reference.









Similar content being viewed by others
References
Rodriguez AL, Williams R, Zhou Y, Lindsley SR, Le U, Grier MD, David Weaver C, Jeffrey Conn P, Lindsley CW (2009) Discovery and SAR of novel mGluR5 non-competitive antagonists not based on an MPEP chemotype. Bioorg Med Chem Lett 19:3209–3213
Eren G, Ünlü S, Nuñez M-T, Labeaga L, Ledo F, Entrena A, Banoğlu E, Costantino G, Şahin MF (2010) Synthesis, biological evaluation, and docking studies of novel heterocyclic diaryl compounds as selective COX-2 inhibitors. Bioorg Med Chem 18:6367–6376
Naresh Kumar R, Poornachandra Y, Nagender P, Mallareddy G, Ravi Kumar N, Ranjithreddy P, Ganesh Kumar C, Narsaiah B (2016) Synthesis of novel trifluoromethyl substituted furo[2,3-b]pyridine and pyrido[3′,2′:4,5]furo[3,2-d]pyrimidine derivatives as potential anticancer agents. Eur J Med Chem 108:68–78
Kawakami K, Takahashi H, Ohki H, Kimura K, Miyauchi S, Miyauchi R, Takemura M (2000) Studies on 8-methoxyquinolones: synthesis and antibacterial activity of 7-(3-amino-4-substituted)pyrrolidinyl derivatives. Chem Pharm Bull 48:1667–1672
Ledoussal B, Bouzard D, Coroneos E (1992) Potent non-6-fluoro-substituted quinolone antibacterials: synthesis and biological activity. J Med Chem 35:198–200
Kotb ER, Soliman HA, Morsy EMH, Abdelwahed NAM (2017) New pyridine and triazolopyridine derivatives: synthesis, antimicrobial and antioxidant evaluation. Acta Pol Pharm 47:861–872
Hu Y, Zhang J, Yu C, Li Q, Dong F, Wang G, Guo Z (2014) Synthesis, characterization, and antioxidant properties of novel inulin derivatives with amino-pyridine group. Int J Biol Macromol 70:44–49
Venkatesh T, Bodke YD, Kenchappa R, Telkar S (2016) Synthesis, antimicrobial and antioxidant activity of chalcone derivatives containing thiobarbitone nucleus. Med Chem (Los Angeles) 6:440–448
Avşar C, Özler H, Berber İ, Civek S (2016) Phenolic composition, antimicrobial and antioxidant activity of Castanea sativa Mill. pollen grains from Black Sea region of Turkey. Int Food Res J 23:1711–1716
Dzoyem JP, Melong R, Tsamo AT, Tchinda AT, Kapche DGWF, Ngadjui BT, McGaw LJ, Eloff JN (2017) Cytotoxicity, antimicrobial and antioxidant activity of eight compounds isolated from Entada abyssinica (Fabaceae). BMC Res Notes 10:118
Jo S-C, Nam K-C, Min B-R, Ahn D-U, Cho S-H, Park W-P, Lee S-C (2006) Antioxidant activity of Prunus mume extract in cooked chicken breast meat. Int J Food Sci Technol 41:15–19
Akinmoladun AC, Ibukun EO, Afor E, Obuotor EM, Farombi EO (2007) Phytochemical constituent and antioxidant activity of extract from the leaves of Ocimum gratissimum. Sci Res Essays 2:163–166
Liu W, Fu Y-J, Zu Y-G, Tong M-H, Wu N, Liu X-L, Zhang S (2009) Supercritical carbon dioxide extraction of seed oil from Opuntia Dillenii Haw and its antioxidant activity. Food Chem 114:334–339
Al-Refai M (2015) Synthesis, characterization and biological evaluation of new 4-Aryl-6-(2,5-dichlorothiophen-3-yl)-1,2-dihydro-2-oxopyridine-3-carbonitrile. Asian J Chem 27:725–728
Ibrahim MM (2015) One-pot synthesis, characterization and antimicrobial activity of new 3-cyano-4-alkyl-6-(2,5-dichlorothiophen-3-yl)-2(1H)-pyridones. Jordan J Chem 10:98–107
Stoe & Cie (2016) X-Area LANA. Stoe & Cie GmbH, Darmstadt, Germany
Sheldrick GM (2014) SHELXT - Integrated space-group and crystal-structure determination. Universität Göttingen, Göttingen, Germany
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Cryst C71:3–8
Stoe & Cie (2016) X-Area Pilatus3_SV, in, Stoe & Cie GmbH, Darmstadt, Germany
Stoe & Cie (2015) X-Area Recipe. Stoe & Cie GmbH, Darmstadt, Germany
Stoe & Cie (2016) X-Area Integrate. Stoe & Cie GmbH, Darmstadt, Germany
Putz H, Brandenburg K (2014) Diamond - crystal and molecular structure visualization. Crystal Impact, Bonn, Germany
Hübschle CB, Sheldrick GM, Dittrich B (2011) ShelXle: a Qt graphical user interface for SHELXL. J Appl Crystallogr 44:1281–1284
Ayoola GA, Folawewo AD, Adesegun SA, Abioro OO, Adepoju-Bello AA, Coker HAB (2008) Phytochemical and antioxidant screening of some plants of apocynaceae from South West Nigeria. African J Plant Sci 2:124–128
Jotani MM, Patel UH, Shah HC (2006) Ethyl 3-Amino-6-phenyl-4-tolylfuro[2,3-b]pyridine-2-carboxylate. Acta Cryst E62:o4906–o4908
Vrabel V, Svorc L, Bradiakova I, Kozisek J, Krutosikova A (2007) 2-[3-(Trifluoromethyl)phenyl]furo[2,3-c]pyridine. Acta Cryst E63:o4516
Garudachari B, Islor AM, Vijesh AM, Gerber T, Hosten E, Betz R (2012) Ethyl 3-(2-ethoxy-2-oxoethoxy)-6-(trifluoromethyl)furo[3,2-c]quinoline-2-carboxylate. Acta Cryst E68:o3389–o3390
Chen D-Q, Tu S-J (2007) 3-Methyl-1,4-diphenyl-1H,5H,7H-furo[3,4-b]pyrazolo[4,3-e]pyridin-5-one. Acta Cryst E63:o1777–o1778
Rybakov VB, Babaev EV, Paronikyan EG (2017) X-ray mapping in heterocyclic design: 18. X-ray diffraction study of a series of derivatives of 3-cyanopyridine-2-one with annelated heptane and octane cycles. Crystallogr Rep 62:219–231
Bernstein J, Davis RE, Shimoni L, Chang N-L (1995) Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew Chem Int Ed Eng 34:1555–1573
Lechel T, Dash J, Brüdgam I, Reißig H-U (2008) Novel furo-pyridine derivatives via Sonogashira Reactions of functionalized pyridines. Eur J Org Chem 2008:3647–3655
Lechel T, Dash J, Eidamshaus C, Brudgam I, Lentz D, Reissig H-U (2010) A three-component synthesis of [b]-alkoxy-[b]-keto-enamides-flexible precursors for 4-hydroxypyridine derivatives and their palladium-catalysed reactions. Org Biomol Chem 8:3007–3014
Al-Ansari IAZ (2016) Effects of structure and environment on the spectroscopic properties of (3-amino-substituted-thieno[2,3-b] pyridine-2-yl)pyridine/quinolin-2-yl)(phenyl)methanones: experimental and theoretical study. J Fluoresc 26:821–834
Acknowledgements
The authors are grateful to Al Al-Bayt University (Mafraq, Jordan). The authors would like also to thank Professor Read Ghanem for his help in running the UV-visible spectra.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
CCDC 1816780 contains crystallographic data for compound III. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/getstructures, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (44) 1223–336,033; or e-mail: deposit@ccdc.cam.ac.uk. (DOCX 676 kb)
Rights and permissions
About this article
Cite this article
Ibrahim, M.M., Al-Refai, M., Al-Fawwaz, A. et al. Synthesis of Fluorescent 1-(3-Amino-4-(4-(tert-butyl)phenyl)−6-(p-tolyl)furo[2,3-b]pyridin-2-yl)ethan-1-one: Crystal Structure, Fluorescence Behavior, Antimicrobial and Antioxidant Studies. J Fluoresc 28, 655–662 (2018). https://doi.org/10.1007/s10895-018-2227-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-018-2227-2