Abstract
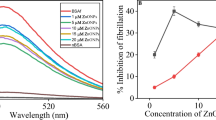
The propensity of native state to form aggregated and fibrillar assemblies is a hallmark of amyloidosis. Our study was focused at analyzing the aggregation and fibrillation tendency of cytochrome c in presence of an organic solvent i.e. acetonitrile. In vitro analysis revealed that the interaction of cytochrome c with acetonitrile facilitated the oligomerization of cytochrome c via the passage through an intermediate state which was obtained at 20 % v/v concentration of acetonitrile featured by a sharp hike in the ANS fluorescence intensity with a blue shift of 20 nm compared to the native state. Oligomers and fibrils were formed at 40 and 50 % v/v concentration respectively as indicated by a significant hike in the ThT fluorescence intensity, red shift of 55 nm in congo red binding assay and an increase in absorbance at 350 nm. They possess β-sheet structure as evident from appearance of peak at 217 nm. Finally, authenticity of oligomeric and fibrillar species was confirmed by TEM imaging which revealed bead like aggregates and a meshwork of thread like fibrils respectively. It could be suggested that the fibrillation of bovine cytchrome c could serve as a model protein to unravel the general aggregation and fibrillation pattern of heme proteins.

ᅟ







Similar content being viewed by others
References
Hamada D, Dobson CM (2002) A kinetic study of β-lactoglobulin amyloid fibril formation promoted by urea. Prot Sci 11:2417–2426
Fändrich M, Schmidt M, Grigorieff N (2011) Recent progress in understanding Alzheimer's beta-amyloid structures. Trends Biochem Sci 36:338–345
Eichner T, Radford SE (2011) A diversity of assembly mechanisms of a generic amyloid fold. Mol Cell 43:8–18
Hamley IW (2012) The amyloid beta peptide: a chemist's perspective. Role in Alzheimer's and fibrillization. Chem Rev 112:5147–5192
Fazili NA, Bhat WF, Naeem A (2014) Induction of amyloidogenicity in wild type HEWL by a dialdehyde: analysis involving multi-dimensional approach. Int J Biol Macromol 64:36–44
Gopalswamy M, Kumar A, Adler J, Baumann M, Henze M, Kumar ST, Fändrich M, Scheidt HA, Huster D, Balbach J (2015) Structural characterization of amyloid fibrils from the human parathyroid hormone. Biochim et Biophys Acta 1854:249–257
Chiti F, Dobson CM (2006) Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 75:333–366
Dobson CM (2003) Protein folding and misfolding. Nature 426:884–890
Ferrao-Gonzales AD, Souto SO, Silva JL, Foguel D (2000) The preaggregated state of an amyloidogenic protein: hydrostatic pressure converts native transthyretin into the amyloidogenic state. Proc Natl Acad Sci U S A 97:6445–6450
Wang SSS, Chen PH, Hung YT (2006) Effects of p-benzoquinone and melatonin on amyloid fibrillogenesis of hen egg-white lysozyme. J Mol Catal B-Enzym 43:49–57
De Groot NS, Ventura S (2005) Amyloid fibril formation by bovine cytochrome c. Spectroscopy 19:199–205
Zaidi S, Hassan MI, Islam A, Ahmad F (2014) The role of key residues in structure, function, and stability of cytochrome c. Cell Mol Life Sci 71:229–255
Pertinheza TA, Boucharda M, Tomlinsonb EJ, Waina R, Fergusonb SJ, Dobson CM, Smith LJ (2001) Amyloid fibril formation by a helical cytochrome. FEBS Lett 495:184–186
Qi PX, Di Stefano DL, Wand AJ (1994) Solution structure of horse heart ferrocytochrome c determined by high-resolution NMR and restrained simulated annealing. Biochemistry 33:64082–66417
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, So¨ ding J, Thompson JD, Higgins DG (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem 31:455–461
Guo Z, Mohanty U, Noehre J, Sawyer TK, Sherman W, Krilov G (2010) Probing the alpha-helical structural stability of stapled p53 peptides: molecular dynamics simulations and analysis. Chem Biol Drug Des 75:348–359
Fazili NA, Naeem A (2015) Anti-fibrillation potency of caffeic acid against an antidepressant induced fibrillogenesis of human α-synuclein: implications for Parkinson's disease. Biochimie 108:178–185
Hudson SA, Ecroyd H, Kee TW, Carver JA (2009) The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS J 276:5960–5972
Kumar S, Udgaonkar JB (2009) Structurally distinct amyloid protofibrils form on separate pathways of aggregation of a small protein. Biochemistry 48:6441–6449
Chen YH, Yang JT, Martinez HM (1972) Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry 11:4120–4131
Naeem A, Khan RH (2004) Characterization of molten globule state of cytochrome c at alkaline, native and acidic pH induced by butanol and SDS. Int J Biochem Cell Biol 36:2281–2292
Vernaglia BA, Huang J, Clark ED (2004) Guanidine hydrochloride can induce amyloid fibril formation from hen egg-white lysozyme. Biomacromolecules 5:1362–1370
Souillac PO, Uversky VN, Millett IS, et al. (2002) Elucidation of the molecular mechanism during the early events in immunoglobulin light chain amyloid fibrillation. Evidence for an off-pathway oligomer at acidic pH. J Biol Chem 277:12666–12679
Hawe A, Sutter M, Jiskoot W (2008) Extrinsic fluorescent dyes as tools for protein characterization. Pharmaceut Res 25:1487–1499
Krebs MRH, Domike KR, Donald AM (2009) Protein aggregation: more than just fibrils. Biochem Soc Trans 37:682–686
Fazili NA, Siddiqui GA, Bhat SA, Afsar M, Furkan M, Naeem A (2015) Rifampicin induced aggregation of ovalbumin: malicious behaviour of antibiotics. Protein Pept Lett 22:644–653
Bhattacharya S, Pandey NK, Roy A, Dasgupta S (2014) Effect of (−)-epigallocatechin gallate on the fibrillation of human serum albumin. Int J Biol Macromol 70:312–319
Naeem A, Bhat SA, Iram A, Khan RH (2016) Aggregation of intrinsically disordered fibrinogen as influence of backbone conformation. Arch Biochem Biophys 603:38–47
Naeem A, Iram A, Bhat SA (2015) Anesthetic 2,2,2-trifluoroethanol induces amyloidogenesis and cytotoxicity in human serum albumin. Int J Biol Macromol 79:726–735
Acknowledgments
The authors are highly thankful for the facilities available at AMU Aligarh. Financial support in the form of an ICMR project, ICMR [45/3/2014-BIO/BMS] is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
No potential conflict of interest was reported by the authors.
Additional information
Mohammad Furkan and Naveed Ahmad Fazili have equal contribution
Rights and permissions
About this article
Cite this article
Furkan, M., Fazili, N.A., Afsar, M. et al. Analysing Cytochrome c Aggregation and Fibrillation upon Interaction with Acetonitrile: an in Vitro Study. J Fluoresc 26, 1959–1966 (2016). https://doi.org/10.1007/s10895-016-1889-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1889-x