Abstract
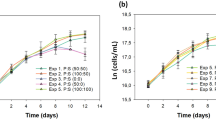
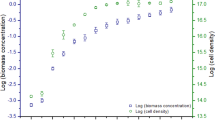
The role of extracellular fatty acids in the interference between two algae, Chlorella vulgaris Beijerink and Pseudokirchneriella subcapitata (Korshikov) Hindak, was assessed by the co-cultivation of the two selected strains, as well as by the chemical analysis of exudates from the culture media of single strain cultures. The effect of culture age and phosphate limitation was evaluated. The experiments showed that the composition and amount of fatty acids, released by C. vulgaris and by P. subcapitata, both in a batch and in a continuous monoculture, depend on the culture age and on the phosphate concentration in the culture medium. We also found that the amount of chlorellin generated in the two algae co-culture increased and was almost exclusively constituted by a mixture of C18 fatty acids. By using the evaluated concentrations of these fatty acids, an artificial chlorellin was prepared. The toxicity of this mixture to P. subcapitata appears to be similar to that of the natural chlorellin. For both algae, a stimulation of growth was observed at low concentrations of the natural chlorellin, whereas higher concentrations produced inhibitory effects on both species. However, P. subcapitata was much more sensitive than C. vulgaris. By using some of these new experimental results, two new mathematical models have been used to describe the toxicity of chlorellin to C. vulgaris and to the interference between C. vulgaris and P. subcapitata, respectively.




Similar content being viewed by others
References
Aliotta, G., Della Greca, M., Monaco, P., Pinto, G., Pollio, A., and Previtera, L. 1990. In vitro algal growth inhibition by phytotoxins of Typha latifolia L. J. Chem. Ecol. 16:2637–2646.
Blum, U., Shafer, S. R., and Lehman, M. E. 1999. Evidence for inhibitory allelopathic interaction involving phenolic acids in field soils: concepts vs. experimental model. Crit. Rev. Plant Sci. 18:673–693.
Bosma, R., Miazek, K., Willemsen, S. M., Vermue, M. H., and Wijffels, R. H. 2008. Growth inhibition of Monodus subterraneus by free fatty acids. Biotech. Bioengin. 101:1108–1114.
Braselton, J. P., and Waltman, P. 2001. A competition model with dynamically allocated inhibitor production. Math. Biosci. 173:55–84.
Chen, C.-Y., Lin, K.-C., and Yang D.-T. 1997. Comparison of the relative toxicity relationships based on batch and continuous algal toxicity tests. Chemosphere 35:1959–1965.
Chiang, I.-Z., Huang, W.-Y., and Wu, J.-T. 2004. Allelochemicals of Botryococcus braunii (Chlorophyceae). J. Phycol. 40:474–480.
Einhellig, F. A. 1996. Interactions involving allelopathy in cropping systems. Agron. J. 88:886–893.
Fergola, P., Aurelio, F., Cerasuolo, M., and Noviello, A. 2004. Influence of mathematical modelling of nutrient uptake and quorum sensing on the allelopathic competitions, in: Proocedings of WASCOM 2003 13th Conference on Waves and Stability in Continuous Media.
Fergola, P., Beretta, E., and Cerasuolo, M. 2006. An allelopathic competition model with quorum sensing and delayed toxicant production. Math. Biosci. Eng. 31:37–50.
Fergola, P., Cerasuolo, M., Pollio, A., Pinto, G., and Della Greca, M. 2007. Allelopathy and competition between Chlorella vulgaris and Pseudokirchneriella subcapitata: Experiments and mathematical model. Ecol. Model. 208:205–214.
Figueredo, C. C., Giani, A., and Bird, D. F. 2007. Does allelopathy contribute to Cylindrospermopsis raciborskii (cyanobacteria) bloom occurrence and geographic expansion? J. Phycol. 43:256–265.
Freedman, H. I., Shukla, J. B., and Takeuchi, Y. 1989. Population diffusion in a two-patch environment. Math. Biosci. 95:111–123.
Gross, E. M. 2003. Allelopathy of aquatic autotrophs. Crit. Rev. Plant Sci. 22:313–339.
Gulley, D. D., Boelter, A. M., and Bergman, H. L. 1989. TOXSTAT 3.0. University of Wyoming, Laramie.
Hallam, T. G., and Ma, Z. 1986. Persistence in population models with demographic fluctuations. J. Math. Biol. 24:327–339.
Hallam, T. G., and Ma, Z. 1987a. Effects of parameter fluctuations on community survival. Math. Biosci. 86:35–49.
Hallam, T. G., and Ma, Z. 1987b. On density and extinction in continuous population models. J. Math. Biol. 25:191–201.
Hallam, T. G., Clark, C. E., and Lassiter, R. R. 1983. Effects of toxicants on populations: a qualitative approach I. Equilibrium environment exposure. Ecol. Model. 18:291–304.
Hsu, S. B., and Waltman, P. 1998. Competition in the chemostat when one competitor produces a toxin. Jpn. J. Ind. Appl. Math. 15:471–490.
Ianora, A., Boersma, M., Casotti, R., Fontana, A., Harder, J., Hoffmann, F., PAVIA, H., POTIN, P., POULET, S. A., and TOTH, G. 2006. New trends in marine chemical ecology. Estuar. Coasts. 29:531–551.
IKAWA, M. 2004. Algal polyunsaturated fatty acids and effects on plankton ecology and other organisms. UNH Cent. Freshwat. Biol. Res. 6:17–44.
Inderjit and Duke, S. O. 2003. Ecophysiological aspects of allelopathy. Planta 217:529–539.
International Standards Organization 1987. Water quality—Algal growth inhibition test. Draft International standard ISO/DIS 8692. Geneva, Switzerland.
LAB Fit Curve Fit Software, Universidade Federal de Campina Grande, Paraíba Brazil 2003.
Lazzara, L., Bianchi, F., Falcucci, M., Hull, V., Modigh, M., and Ribera d’Alcala’, M. 1990. Pigmenti clorofilliani, pp. 207–223, in M. Innamorati, I. Ferrari, D. Marino, M. Ribera D’Alcala’ (eds.). Nova Thalassa, vol.11, Metodi nell’ecologia del plancton marino. Società Italiana di Biologia Marina. Edizioni LINT Trieste.
Macias, F. A. 1995. Allelopathy in the search for natural herbicide models. in Inderrjit, K. M. M. Darkshini, F. A. Einhellig (eds.) Allelopathy: Organisms, Processes and Application: ACS Symposium Series 582 American Chemical Society, Washington, DC.
Macias, F. A., Oliveros-Bastidas, A., Marin, D., Carrera, C., Chinchilla, N., and Molinillo, J. M. G. 2008. Plant biocommunicators: their phytotoxicity, degradation studies and potential use as herbicide models. Phytochem. Rev. 7:179–194.
McCracken, M. D., Middaugh, R. E., and Middaugh, R. S. 1980. A chemical characterization of an algal inhibitor obtained from Chlamydomonas. Hydrobiologia 70:271–276.
Meyer, R. R., and Roth, P. M. 1972. Modified damped least squares: an algorithm for nonlinear estimation. J. Inst. Math. Appl. 9:218–233.
Mukhopadhyay, A., Chattopadhyay, J., and Tapaswi, P. K. 1998. A dealy differential equations model of plankton allelopathy. Math. Biosci. 149:167–189.
Mukhopadhyay, A., Tapaswi, P.K., Chattopadhyay, J. 2003. A space time state-space model of phytoplankton allelopathy. Nonlin. Anal.: Ser. B Real World Appl. 4:437–456.
Murata, H., Sakai, T., Endo, M., Kuroki, A., Kimura, M., and Kumanda, K. 1989. Chattonella marina red tide plankton chemical removal study. Effect of free radicals from hydrogen peroxide and polysaturated fatty acids. Nippon Suisan Gakkaishi 55:1075–1082.
Nichols, H. W. 1973. Growth media. Fresh water, pp. 7–24, in J. R. Stein (ed.). Handbook of Phycological Methods: Cult. Methods Growth Meas. Cambridge University Press, London.
Ohta, S., Shiomi, Y., Kawashima, A., Aozasa, O., Nakao, T., Nagate, T., Kitamura, K., and Miyata, H. 1995. Antibiotic effect of linolenic acid from Chlorococcum strain Hs-101 and Dunaliella primolecta on methicillin-resistant Staphylococcus aureus. J. Appl. Phycol. 7:121–127.
Pollio, A., Pinto, G., Ligrone, R., and Aliotta, G. 1993. Effects of potential allelochemical α-asarone on growth, physiology and ultrastructure of two unicellular green algae. J. Appl. Phycol. 5:395–403.
Pratt, R., and Fong, J. 1940. Studies on Chlorella vulgaris II. Further evidence that Chlorella cells form a growth-inhibiting substance. Am. J. Bot. 27:431–436.
Scutt, J. E. 1964. Autoinhibitor production by Chlorella vulgaris. Am. J. Bot. 51:581–584.
Smith, H. L., and Waltman, P. 1995. The Theory of the Chemostat—Dynamics of Microbial Competition. Cambridge Studies in Mathematical Biology. Cambridge University Press. Cambridge.
Spoehr, H. A., Smith, J. H. C., Strain, H. H., Milner, H. W., and Hardin, G. J. 1949. Fatty Acids Antibacterials from Plants, Publication 586. Carnegie Institution of Washington.
Sushchik, N. N., Kalacheva, G. S., and Gladyshev, M. I. 2001. Secretion of free fatty acids by prokaryotic and eukarotic algae at optimal, sopraoptimal, and suboptimal growth temperatures. Microbiology 70:542–547.
Sushchik, N. N., Kalacheva, G. S., Zhila, M. I., Gladyshev, M. I., and Volova, T. G. 2003. A temperature dependence of the intra- and extracellular fatty-acid composition of green algae and cyanobacterium. Russ. J. Plant Physiol . 50:374–380.
Von Elert, E. and Jüttner, F. 1997. Phosphorus limitation and not light controls the extracellular release of allelophatic compounds by Trichormus doliolum (Cyanobacteria). Limnol. Oceanogr. 42:1796–1802.
Wolfram-Research. Mathematica. www.wolfram.com, 1988.
Wu, J.-T., Chiang, Y.-R., Huang, W.-Y., and Jane, W.-N. 2006. Cytotoxic effects of free fatty acids on phytoplankton algae and cyanobacteria. Aquat. Toxicol. 80:338–345.
Yamada, N., Murakami, N., Morimoto, T., and Sakakibara, J. 1993. Auto-growth inhibitory substances from the freshwater cyanobacterium Phormidium tenue. Chem. Pharmaceut. Bull. 41:1863–1865.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Proof (Lemma 2)
If we set
due to system (7), we get
and by integrating from 0 to t, we obtain
and then
thus proving, for any initial condition, the boundedness of the solutions of (7).
Proof( Theorem 2)
In order to prove the theorem, we look for positive steady state solutions (S* > 0, N* > 0, p* > 0) and we solve the system obtained by setting the right-hand side of system (7) equal to zero. In this way, we get
and
with a few calculations from (14) we find that N* > 0, p* > 0, if and only if S* < 1. We have to exclude the value S* = 1 because it makes both p* and N* equal to zero. Therefore (by omitting the star), we study the solutions of the equation
if we define
then the problem of finding the solution of (15) is changed into finding the solution of h(S) = g(S). Remembering that S < 1, it is easy to show that in the interval [0, 1], h(S) is strictly decreasing whereas g(S) is strictly increasing.
Therefore, because h(1) = 1 and \( g(1) = \frac{m}{{a + 1}} \) the two curves admit only one intersection point with abscissa S* < 1 (Fig. 5).
Here we represent the plots of the two functions h(S) and g(S) defined in (12) in the proof of Theorem 4. These curves, as shown, admit only one intersection point with abscissa S* < 1
Proof (Theorem 3)
-
(i)
The roots of Eq. (9) computed in E 0 = (1, 0, 0) are
Therefore, the steady state E0 turns out asymptotically stable if
-
(ii)
In E* = (S*, N*, p*), Eq. (9) can be written as follows
where
are positive constants. In order to study the stability properties of the equilibrium, we can use the Routh–Hurwitz criterion. We observe that, being
-
1.
\( {a_{11}} + {a_{22}} + {a_{33}} > 0 \)
-
2.
\( \begin{array}{*{20}{c}} {{a_{12}}{a_{21}}{a_{33}} + {a_{11}}{a_{22}}{a_{33}} - {a_{12}}{a_{23}}{a_{31}} + {a_{11}}{a_{23}}{a_{32}} - {a_{13}}{a_{22}}{a_{31}} - {a_{13}}{a_{21}}{a_{32}}} \hfill \\ { = \frac{{ - \left( {1 - {S^* }} \right)\left( { - \frac{{a + ak + k{S^{ * 2}}}}{{{S^* }\left( {a + {S^* }} \right)}} - 2k{p^* }\left( {{p^* } + 2{N^* }} \right)} \right)}}{\alpha } > 0} \hfill \\ \end{array} \)
-
3.
\( \begin{array}{*{20}{c}} {\left( {{a_{11}} + {a_{22}} + {a_{33}}} \right)\left( {{a_{12}}{a_{21}} + {a_{11}}{a_{22}} - {a_{13}}{a_{31}} + {a_{23}}{a_{32}} + {a_{11}}{a_{33}} + {a_{22}}{a_{33}}} \right)} \hfill \\ { - \left( {{a_{12}}{a_{21}}{a_{33}} + {a_{11}}{a_{22}}{a_{33}} - {a_{12}}{a_{23}}{a_{31}} + {a_{11}}{a_{23}}{a_{32}} + - {a_{13}}{a_{22}}{a_{31}} - {a_{13}}{a_{21}}{a_{32}}} \right) > 0} \hfill \\ \end{array} \)
then all the hypotheses of the Routh–Hurwitz criterion are satisfied and the local stability of the equilibrium E* follows.
Rights and permissions
About this article
Cite this article
DellaGreca, M., Zarrelli, A., Fergola, P. et al. Fatty Acids Released by Chlorella vulgaris and Their Role in Interference with Pseudokirchneriella subcapitata: Experiments and Modelling. J Chem Ecol 36, 339–349 (2010). https://doi.org/10.1007/s10886-010-9753-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-010-9753-y