Abstract
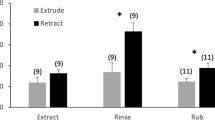
Mated female Heliothis virescens and H. subflexa were induced to produce sex pheromone during the photophase by injection of pheromone biosynthesis activating neuropeptide (PBAN). When injected with 1 pmol Hez-PBAN, the total amount of pheromone that could be extracted from glands of mated females during the photophase was similar to that extracted from virgin females in the scotophase. The PBAN-induced profile of pheromone components was compared between mated, PBAN-injected females and virgin females during spring and fall. Virgin females exhibited some differences in the relative composition of the pheromone blend between spring and fall, but no such temporal differences were detected in PBAN-injected, mated females. Because the temporal variation in pheromone blend composition was greater for virgin females than for PBAN-injected females, PBAN can be used to determine a female’s native pheromone phenotype. This procedure has the advantages that pheromone glands can be extracted during the photophase, from mated females that have already oviposited.
Similar content being viewed by others
References
Abernathy, R. L., Teal, P. E. A., Meredith, J. A., and Nachman, R. J. 1996. Induction of pheromone production in a moth by topical application of a pseudopeptide mimic of a pheromonotropic neuropeptide. Proc. Natl. Acad. Sci. USA 93:12621–12625.
Arima, R., Takahara, K., Kadoshima, T., Numazaki, F., Ando, T., Uchiyama, M., Nagasawa, H., Kitamura, A., and Suzuki, A. 1991. Hormonal regulation of pheromone biosynthesis in the silkworm moth, Bombyx mori (Lepidoptera: Bombycidae). Appl. Entomol. Zool. 26:37–147.
Bjostad, L. B. and Roelofs, W. L. 1983. Sex pheromone biosynthesis in Trichoplusia ni: key steps involve delta-11 desaturation and chain-shortening. Science 220:1387–1389.
Boos, D. D. and Brownie, C. 1989. Bootstrap methods for testing homogeneity of variances. Technometrics 31:69–82.
Butlin, R. 1995. Genetic variation in mating signals and responses, pp. 327–366, D. M. Lambert and H. G. Spencer (eds.). Speciation and the Recognition Concept: Theory and Application. Johns Hopkins Univ. Press, Baltimore, MD.
Choi, M.-Y., Han, K. S., Boo, K. S., and Jurenka, R. A. 2002. Pheromone biosynthetic pathways in the moths Helicoverpa zea and Helicoverpa assaulta. Insect Biochem. Mol. Biol. 32:1353–1359.
Conover, W. J., Johnson, M. E., and Johnson, M. M. 1981. A comparative study of tests for homogeneity of variances, with applications to the outer continental shelf bidding data. Technometrics 23:351–361.
Coyne, J. A. and Orr, H. A. 1998. The evolutionary genetics of speciation. Phil. Trans. R. Soc. London Series B: Biol. Sci. 353:287–305.
Fabriàs, G., Marco, M.-P., and Camps, F. 1994. Effect of the pheromone biosynthesis activating neuropeptide on sex pheromone biosynthesis in Spodoptera littoralis isolated glands. Arch. Insect Biochem. Physiol. 27:77–87.
Heath, R. R., Mclaughlin, J. R., Prosholt, F., and Teal, P. E. A. 1991. Periodicity of female sex pheromone titer and release in Heliothis subflexa and H. virescens (Lepidoptera: Noctuidae). Ann. Ent. Soc. Am. 84:182–189.
Jurenka, R. A. 1996. Signal transduction in the stimulation of sex pheromone biosynthesis in moths. Arch. Insect Biochem. Physiol. 33:245–258.
Klun, J. A., Plimmer, J. R., Bierl-Leonhardt, B. A., Sparks, A. N., Primiani, M., Chapman, O. L., Lee, G. H., and Lepone, G. 1980a. Sex pheromone chemistry of female corn earworm moth, Heliothis zea. J. Chem. Ecol. 6:165–175.
Klun, J. A., Bierl-Leonhardt, B. A., Plimmer, J. R., Sparks, A. N., Primiani, M., Chapman, O. L., Lepone, G., and Lee, G. H. 1980b. Sex pheromone chemistry of the female tobacco budworm moth Heliothis virescens. J. Chem. Ecol. 6:177–183.
Klun, J. A., Leonardt, B. A., Lopez, J. D., and Lachance, L. E. 1982. Female Heliothis subflexa (Lepidoptera, Noctuidae) sex pheromone — chemistry and congeneric comparisons. Environ. Entomol. 11:1084–1090.
Kurtti, T. J. and Brooks, M. A. 1976. The dissociation of insect embryos for cell culture. In Vitro 12: 141–146.
Laster, M. L. 1972. Interspecific hybridization of Heliothis virescens and H. subflexa. Environ. Entomol. 1:682–687.
Linn, C. E. Jr. and Roelofs, W. L. 1995. Pheromone communication in moths and its role in the speciation process, pp. 263–300, D. M. Lambert and H. G. Spencer (eds.). Speciation and the Recognition Concept: Theory and Application. Johns Hopkins Univ. Press, Baltimore, MD.
Löfstedt, C. 1990. Population variation and genetic control of pheromone communication systems in moths. Entomol. Exp. Appl. 54:199–218.
Löfstedt, C. 1993. Moth pheromone genetics and evolution. Phil. Trans. R. Soc. London Series B: Biol. Sci. 340:167–177.
Morse, D. and Meighen, E. 1986. Pheromone biosynthesis and role of functional groups in pheromone specificity. J. Chem. Ecol. 12:335–351.
Ozawa, R. A., Ando, T., Nagasawa, H., Kataoka, H., and Suzuki, A. 1993. Reduction of the acyl group: the critical step in bombykol biosynthesis that is regulated in vitro by the neuropeptide hormone in the pheromone gland of Bombyx mori. Biosci. Biotech. Biochem. 57:2144–2147.
Phelan, P.L. 1997. Evolution of mate signalling in moths: phylogenetic considerations and predictions from the asymmetric tracking hypothesis, pp. 240–256, J. Choe and B. Crespi (eds.). Evolution of Mating Systems in Insects and Arachnids. Cambridge University Press, Cambridge, UK.
Pope, M. M., Gaston, L. K., and Baker, T. C. 1982. Composition, quantification, and periodicity of sex pheromone gland volatiles from individual Heliothis virescens females. J. Chem. Ecol. 8:1043–1055.
Pope, M. M., Gaston, L. K., and Baker, T. C. 1984. Composition, quantification, and periodicity of sex pheromone volatiles from individual Heliothis zea females. J. Insect Physiol. 30:943–945.
Prosholt, F. I. and Lachance, L. E. 1974. Analysis of sterility in hybrids from interspecific crosses between Heliothis virescens and H. subflexa. Ann. Entomol. Soc. Am. 67:445–449.
Raina, A. K., Jaffe, H., Kempe, T. G., Keim, P., Blacher, R. W., Fales, H. M., Riley, C. T., Klun, J. A., Ridgway, R., and Hayes, D. K. 1989. Identification of a neuropeptide hormone that regulates pheromone production in female moths. Science 244:796–798.
Rafaeli, A. 2002. Neuroendocrine control of pheromone biosynthesis in moths. Int. Rev. Cytol. 213: 49–91.
Rafaeli, A. and Jurenka, R. A. 2003. PBAN regulation of pheromone biosynthesis in female moths, pp. 107–136, G. J. Blomquist and R. Vogt (eds.). Insect Pheromones – Biochemistry and Molecular Biology. Academic Press, New York, NY.
Ramaswamy, S. B., Randle, S. A., and Ma, W. K. 1985. Field evaluation of the sex pheromone components of Heliothis virescens (Lepidoptera: Noctuidae) in cone traps. Environ. Entomol. 14:293–296.
Roelofs, W. L. and Wolf, W. A. 1988. Pheromone biosynthesis in Lepidoptera. J. Chem. Ecol. 14:2019–2031.
Roelofs, W. L., Hill, A. S., Cardé, R. T., and Baker, T. C. 1974. Two sex pheromone components of the tobacco budworm moth, Heliothis virescens. Life Sci. 14:1555–1562.
Sas. 2002. The SAS System for Windows. Release 8.03. SAS Institute, Cary NC.
Sheck, A. L. and Gould, F. 1993. The genetic basis of host range in Heliothis virescens: larval survival and growth. Entomol. Exp. Appl. 69:157–172.
Sheck, A. L. and Gould, F. 1995. Genetic analysis of differences in oviposition preferences of Heliothis virescens and H. subflexa (Lepidoptera: Noctuidae). Environ. Entomol. 24:341–347.
Sheck, A. L. and Gould, F. 1996. The genetic basis of differences in growth and behavior of specialist and generalist herbivore species: selection on hybrids of Heliothis virescens and Heliothis subflexa (Lepidoptera). Evolution 50:831–841.
Teal, P. E. A. and Oostendorp, A. 1995a. Production of pheromone by hairpencil glands obtained from interspecific hybridization between Heliothis virescens and H. subflexa. J. Chem. Ecol. 21:59–67.
Teal, P. E. A. and Oostendorp, A. 1995b. Effect of interspecific hybridization between Heliothis virescens and H. subflexa (Lepidoptera: Noctuida) on sex pheromone production by females. J. Insect Physiol. 41:519–525.
Teal, P. E. A. and Tumlinson, J. H. 1987. The role of alcohols in pheromone biosynthesis by two noctuid moths that use acetate pheromone components. Arch. Insect Biochem. Physiol. 4:261–269.
Teal, P. E. A. and Tumlinson, J. H. 1997. Effects of interspecific hybridization between Heliothis virescensand Heliothis subflexa on the sex pheromone communication system, pp. 535–547, R. T. Cardé and A. K. Minks (eds.). Insect Pheromone Research, New Directions. Chapman & Hall, New York, NY.
Teal, P. E. A., Heath, R. R., Tumlinson, J. H., and Mclaughlin, J. R. 1981. Identification of sex pheromone of Heliothis subflexa (G.) (Lepidoptera: Noctuidae) and field trapping studies using different blends of components. J. Chem. Ecol. 7:1011–1022.
Teal, P. E. A., Tumlinson, J. H., Mclaughlin, J. R., Heath, R., and Rush, R. A. 1984. ($Z)$-11-Hexadecen-1-ol: a behavioral modifying chemical present in the pheromone gland of female Heliothis zea (Lepidoptera: Noctuidae). Can. Entomol. 116:777–779.
Teal, P. E. A., Tumlinson, J. H., and Heath, R. R. 1986. Chemical and behavioral analyses of volatile sex pheromone components released by calling Heliothis virescens (F.) females (Lepidoptera: Noctuidae). J. Chem. Ecol. 12:107–125.
Teal, P. E. A., Tumlinson, J. H., and Oostendorp, A. 1989. Enzyme-catalyzed pheromone synthesis by Heliothis moths, pp. 332–343, J. R. Whitaker and P. E. Sonnet (eds.). Biocatalysis in Agricultural Biotechnology. American Chemical Society, Washington, D.C.
Teal, P. E. A., Oostendorp, A., and Tumlinson, J. H. 1993. Induction of pheromone production in females of Heliothis virescens (F.) and H. subflexa (Gn.) (Lepidoptera: Noctuidae) during the photophase. Can. Entomol. 125:355–366.
Tillman, J. A., Seybold, S. J., Jurenka, R. A., and Blomquist, G. J. 1999. Insect pheromones–an overview of biosynthesis and endocrine regulation. Insect Biochem. Mol. Biol. 29:481–514.
Tumlinson, J. H., Hendricks, P. E., Mitchell, E. R., Doolittle, R. E., and Brennan, M. M. 1975. Isolation, identification and synthesis of the sex pheromone of the tobacco budworm . J. Chem. Ecol. 1:203–214.
Tumlinson, J. H., Heath, R. R., and Teal, P. E. A. 1982. Analysis of chemical communication systems of Lepidoptera, pp. 1–25, B. A. Leonhardt and M. Beroza (eds.). Insect Pheromone Technology—Chemistry and Applications. American Chemical Society, Washington D.C.
Vetter, R. S. and Baker, T. C. 1984. Behavioral responses of male Heliothis zea moths in sustained flight-tunnel to combinations of four compounds identified from female sex pheromone gland. J. Chem. Ecol. 10:193–202.
Vickers, N. J. 2002. Defining a synthetic blend attractive to male Heliothis subflexa under wind tunnel conditions. J. Chem. Ecol. 28:1255–1267.
Vickers, N. J., Christensen, T. A., Mustaparta, H., and Baker, T. C. 1991. Chemical communication in heliothine moths III. Flight behavior of male Helicoverpa zea and Heliothis virescens in response to varying ratios of intra- and interspecific sex pheromone components. J. Comp. Physiol. A 169:275–280.
Wolf, W. A. and Roelofs, W. L. 1989. Enzymes involved in the biosynthesis of sex pheromones in moths, pp. 323–331, J. R. Whitaker and P. E. Sonnet (eds.). Biocatalysis in Agricultural Biotechnology. American Chemical Society, Washington, D.C.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
GROOT, A.T., FAN, Y., BROWNIE, C. et al. EFFECT OF PBAN ON PHEROMONE PRODUCTION BY MATED Heliothis virescens AND Heliothis subflexa FEMALES. J Chem Ecol 31, 15–28 (2005). https://doi.org/10.1007/s10886-005-0970-8
Issue Date:
DOI: https://doi.org/10.1007/s10886-005-0970-8