Abstract
The risk of pulmonary complications is high after major abdominal surgery but may be reduced by prophylactic postoperative noninvasive ventilation using continuous positive airway pressure (CPAP). This study compared the effects of intermittent mask CPAP (ICPAP) and continuous helmet CPAP (HCPAP) on oxygenation and the risk of pulmonary complications following major abdominal surgery. Patients undergoing open abdominal aortic aneurysm repair or pancreaticoduodenectomy were randomized (1:1) to either postoperative ICPAP or HCPAP. Oxygenation was evaluated as the partial pressure of oxygen in arterial blood fraction of inspired oxygen ratio (PaO2/FIO2) at 6 h, 12 h, and 18 h postoperatively. Pulmonary complications were defined as X-ray verified pneumonia/atelectasis, clinical signs of pneumonia, or supplementary oxygen beyond postoperative day 3. Patient-reported comfort during CPAP treatment was also evaluated. In total, 96 patients (ICPAP, n = 48; HCPAP, n = 48) were included, and the type of surgical procedure were evenly distributed between the groups. Oxygenation did not differ between the groups by 6 h, 12 h, or 18 h postoperatively (p = 0.1, 0.08, and 0.67, respectively). Nor was there any difference in X-ray verified pneumonia/atelectasis (p = 0.40) or supplementary oxygen beyond postoperative day 3 (p = 0.53). Clinical signs of pneumonia tended to be more frequent in the ICPAP group (p = 0.06), yet the difference was not statistically significant. Comfort scores were similar in both groups (p = 0.43), although a sensation of claustrophobia during treatment was only experienced in the HCPAP group (11% vs. 0%, p = 0.03). Compared with ICPAP, using HCPAP was associated with similar oxygenation (i.e., PaO2/FIO2 ratio) and a similar risk of pulmonary complications. However, HCPAP treatment was associated with a higher sensation of claustrophobia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Postoperative pulmonary complications (PPCs) frequently occur after major abdominal surgery (defined as abdominal surgery requiring laparotomy) and are associated with increases in morbidity, length of hospital stay, and mortality rate [1, 2]. PPCs can be broadly defined as pulmonary symptoms affecting the treatment course (some examples include: respiratory failure requiring intensive care, pneumonia, atelectasis requiring bronchoscopy, PaO2/FiO2 < 300), bronchospasm [newly detected expiratory wheezing] and pleural effusion or pneumothorax reguirering surgical intervention caused by inadequate lung re-expansion) [1, 2]. However, no consensus regarding the definition of PPCs has been reached [1, 2]. Therefore, the incidence of PPCs varies considerably (9–40%), depending on the definition applied [1,2,3].
Prophylactic use of continuous positive airway pressure (CPAP) in the postoperative period may reduce the risk of PPCs [4,5,6]. CPAP increases functional residual capacity and vital capacity, enhancing gas exchange and oxygenation while reducing respiratory work [7]. CPAP can be delivered through a nasal airway, face mask, or a helmet interface [8]. A meta-analysis by Ferreyra et al. [4] concluded that for CPAP to decrease the risk of PPCs, a continuous treatment period of at least 6 h is recommended [4]. Although CPAP may be administered via a face mask over an extended period, this modality is poorly tolerated by patients and is time-consuming for staff in the postanesthesia care unit (PACU) [9]. Therefore, the standard mask CPAP treatment is commonly applied intermittently in the PACU: for example, for 10–30 min every 2–3 h [5]. Because patients can generally tolerate the helmet better than the mask interface [10], postoperative treatment durations may be prolonged by using the helmet, perhaps preventing PPCs more effectively after major abdominal surgery. However, to our knowledge, these CPAP delivery modalities have not been compared previously in a randomized trial.
We, therefore, examined the effects of continuous helmet CPAP (HCPAP) versus intermittent mask CPAP (ICPAP) on postoperative oxygenation and the risk of pulmonary complications following major abdominal surgery.
2 Methods
This single-center single-blinded randomized controlled trial was approved by the Regional ethical committees of the capital region of Denmark (H-1-2013-094) and registered at clinicaltials.gov (NCT02173327). This manuscript adheres to the applicable CONSORT guidelines, and all patients provided written informed consent before enrollment.
2.1 Patient population
Patients aged over 18 years, scheduled for major abdominal surgery (i.e., Whipple’s procedure or open abdominal aortic aneurism repair), with a planned extended overnight stay (standard care) in the PACU were eligible for inclusion. Patients’ postoperative pulmonary risk scores were evaluated using the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) score [11]. Patients were not eligible for inclusion in the case of severe pulmonary disease (i.e., need for supplementary oxygen at home), upper airway deformities, CPAP at home, claustrophobia, or were unable to provide consent. Other exclusion criteria were: inoperability, operative duration > 6 h (due to logistical reasons and availability of the primary investigator), re-operation, admission to the intensive care unit (ICU) from the operating room, and non-epidural anesthesia.
2.2 Randomization and blinding
Patients were randomized into two groups (1:1) using a computer-generated randomization list (randomizer.org) to receive either HCPAP or conventional ICPAP. Allocation was concealed in numbered sealed opaque envelopes, which were opened by a research nurse when each patient arrived in the PACU after surgery. Neither the patient nor the PACU staff were blinded to treatment allocation. However, the investigator conducting the statistical analysis was blinded to randomization.
2.3 Study protocol
All patients were anesthetized in accordance with the standard departmental protocol for the specified surgical procedure. Before induction, an epidural catheter was inserted into the 7–9th thoracic epidural space. A total of 5 mL 0.5% bupivacaine was administered every 75 min during surgery. Thereafter, 5 mL/h of 2.5 mg/mL bupivacaine was administered until postoperative day 3. Propofol, remifentanil, and cisatracurium were used to induce general anesthesia, which was maintained using either propofol/remifentanil or sevoflurane/remifentanil. Neuromuscular blockade was achieved by cisatracurium and monitored by neuromuscular train of four (TOF) stimulation. Extubation was only performed when the TOF ratio was > 90%.
In the PACU, a Comfort CPAP Zip helmet (DIMAR, Medolla, Italy) was connected to a dedicated CF 800 CPAP apparatus (Dräger, Lübeck, Germany) and fitted to each patient in the HCPAP group. A pressure release valve was adjusted to ensure CPAP at 7.5 cm H2O [12, 13] while the oxygen/atmospheric air mixture and flow were titrated to achieve oxygen saturation > 92% or a target set by the attending anesthesiologist. HCPAP was administered continuously for 6 h, although small breaks were permitted if the patient experienced nausea or claustrophobia. For each patient in the ICPAP group, treatment was initiated by fitting a CPAP mask connected to a dedicated CF 800 CPAP apparatus (Dräger) with the pressure release valve set to 10 cm H2O as per standard treatment at the PACU. ICPAP was administered for 10 min every 2 h throughout the patient’s PACU stay except during the night when a continuous 4-h break was allowed to help the patient sleep.
For both groups, CPAP treatment was initiated as soon as possible after the patient arrived in the PACU after ruling out acute pain, nausea, or circulatory problems. The number, duration, and cause of CPAP breaks were noted for each patient. There were no restrictions on patient posture during HCPAP treatment, but ICPAP was administered with each patient lying on their back with a backrest tilted to 45 degrees. Postoperative was assessed using a numeric rating scale (NRS; 0–10) and treated with epidural boluses or IV opioids if pain exceeded NRS 3 during rest or five during movement. Discharge from the PACU was according to a modified Aldrete discharge score [14].
2.4 Outcome measures
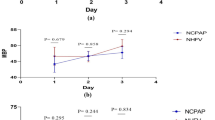
The primary outcome was the partial pressure of oxygen in arterial blood (PaO2)/fraction of inspired oxygen (FIO2) ratio, measured 6 h, 12 h, and 18 h after arrival in the PACU. PaO2 was measured by drawing 2 mL of blood from the arterial cannula and using a point-of-care ABL 710 blood gas analyzer (Radiometer, Brønshøj, Denmark), whereas the FIO2 was read from the CPAP apparatus.
Secondary outcome measures were mortality within 30 days, length of PACU stay, and the frequency of PPCs (defined as the need for supplementary oxygen for more than 3 days, signs of pneumonia [coughing, profusion of mucus, shortness of breath, chest pain, temperature > 38 °C, heart rate > 100 beats per min, or a decrease in oxygen saturation], ICU admission due to respiratory insufficiency, or X-ray verified (by a radiologist) pneumonia/atelectasis within 6 days after surgery). Each patient rated their overall comfort during CPAP treatment using a numeric rating scale (NRS, 0–10) 2 h after PACU arrival and before PACU discharge. In addition to overall comfort, sensations of claustrophobia (y/n), pressure (y/n), dryness (y/n), and smell (y/n) were also recorded.
2.5 Sample size calculation
Based on a previous helmet CPAP study [15], 92 patients (46 in each group) were required to detect a difference in PaO2/FIO2 ratio of 50 mm Hg between groups with a power (1-beta) of 0.8 and a significance level (alpha) of 0.05. To allow for a 10% dropout rate, 102 patients had to be included.
2.6 Statistical analysis
The underlying distribution of all variables was determined using visual inspection of histograms and the Kolmogorov–Smirnov test. Continous data are reported as means (± standard deviation) or medians (interquartile range), and group differences were evaluated using either Student’s t test/standardized difference or the Mann–Whitney U test, depending on the distribution. Differences in proportions were evaluated using the chi-square test or Fisher’s exact test as appropriate. All analyses were performed in SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA) and a p value < 0.05 was considered statistically significant.
3 Results
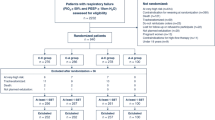
Inclusion was initiated in November 2013 and completed in August 2016. A total of 380 patients were screened for trial participation, and 137 patients were randomized (Fig. 1). After randomization, 41 patients were excluded due to: inoperability (n = 15), operative duration > 6 h (n = 12), re-operation (n = 3), admission to ICU (n = 2), non-epidural anesthesia (n = 2), withdrawal of consent (n = 2), intraoperative mortality (n = 1), and loss to follow-up (n = 4). Thus, 96 patients were included for analysis and randomization ensured that the types of surgical procedure were evenly distributed between the groups (Table 1).
3.1 Baseline characteristics
Baseline characteristics for the study cohort are presented in Table 1. Participants in the mask and helmet groups were similar with regard to age, body mass index, American Society of Anesthesiologists physical status score, smoking status, and level of alcohol consumption. There were no significant differences between the postoperative pulmonary risk scores (i.e., ARISCAT scores [11]) from each group.
3.2 Primary outcome
There were no statistically significant differences in PaO2/FIO2 ratios between treatment groups at 6 h, 12 h, and 18 h postoperatively (Table 2).
3.3 Secondary outcomes
Postoperative outcomes and comfort data are presented in Tables 3 and 4, respectively. There were no differences between the groups in terms of the need for supplementary oxygen for more than 3 days (p = 0.39), pneumonia verified by chest X-ray (p = 0.40), length of PACU stay (p = 0.18), ICU admission due to respiratory insufficiency (p = 0.53), or 30-day mortality. Signs of pneumonia tended to be more frequent in the ICPAP group (p = 0.06), yet the difference was not statistically significant.
There was no difference in the reported level of patient comfort between the two groups after 2 h of treatment (p = 0.43). Five patients (11%) in the helmet group and none in the mask group reported a claustrophobic sensation at 2 h after the initiation of CPAP treatment (p = 0.03). There were no significant differences in sensations of unpleasantness, pressure (p = 0.14), dryness (p = 0.34), or smell (p = 0.60). The helmet treatment was briefly paused in 26 of 48 (58%) patients (median, 1.5 min [range, 0–9 min]), whereas all patients given mask CPAP tolerated the treatment without breaks. The mean total treatment durations were 358 min (range, 350–360 min) for the helmet interface and 70 min (range, 66–80) for the mask interface.
4 Discussion
This study compared HCPAP with ICPAP treatment of patients recovering from major abdominal surgery. We found no difference between the modalities in the risk of pulmonary complications or the level of postoperative oxygenation measured using the PaO2/FIO2 ratio. The patients showed similar tolerance to both treatments.
Several studies have demonstrated the benefits of prophylactic and therapeutic CPAP compared with respiratory physiotherapy and oxygen therapy following abdominal surgery (e.g., shorter hospital stays, fewer complications, and reduced morbidity) [4,5,6]. However, a recent report contradicted these findings and presented no difference in postoperative morbidity between CPAP or standard postoperative care [16]. However, this report included a broader definition of major surgery than that of the current study and a shorter treatment length (at least 4 h) than that presented by Ferreyra et al. [4]. Also, the report did not include a comparison between CPAP modalities. Hence, comparisons between helmet and mask CPAP treatments (in contrast to noninvasive positive pressure ventilation) in clinical settings are still lacking, particularly in the postoperative period.
In our study, there were no significant differences between the groups in the PaO2/FIO2 ratios measured before or after CPAP treatment (Table 2). Interestingly, at 18 h, the helmet treatment had been discontinued for 12 h, and yet the PaO2/FIO2 ratio did not differ significantly from that measured in patients treated with ICPAP. In patients with acute respiratory failure (ARF), CPAP delivered via the helmet resulted in similar improvements in PaO2/FIO2 ratios to those observed in patients who had received CPAP via the mask interface [17, 18]. However, in one study, by 12 h after the initiation of treatment, only the helmet group had a PaO2/FIO2 ratio that was significantly higher than that recorded at baseline [17]. In addition, in infants with bronchiolitis, CPAP delivered via the helmet or mask interface resulted in a similar improvement in PaO2/FIO2 ratio [19]. In a physiological study, the helmet was reported as efficient as the mask interface in delivering CPAP to healthy adult volunteers [20]. However, due to CO2 rebreathing, the helmet interface requires a greater flow of fresh gas to maintain PaCO2 levels [15, 20, 21]. Although few studies have compared the efficacy of using a helmet or mask interface to deliver CPAP, these have consistently reported similar improvements in PaO2/FIO2 ratio for both methods.
In the current study, the frequency of clinical signs of pneumonia/atelectasis (e.g., coughing, profusion of mucus, shortness of breath, chest pain, temperature > 38 °C, heart rate > 100 beats per min, or a decrease in oxygen saturation) tended to be higher in the mask group (p = 0.06) but this did not reach statistics significance and there was no difference in the rates of X-ray verified pneumonia/atelectasis between the groups. Prophylactic CPAP in the postoperative period is reportedly associated with a decreased risk of atelectasis and improved spirometric data, compared with respiratory and oxygen therapy [7, 13, 22]. However, few studies have compared CPAP delivery interfaces and is possible that our study was underpowered with regard to these secondary outcomes.
We found no significant difference in the rates of ICU admission due to respiratory failure between patients who used the helmet versus the mask interface (10% vs. 6%, respectively; p = 0.46; Table 3). Our study was not conducted on patients with ARF. Therefore, the rate of ICU admission due to respiratory failure was low, and the study was not powered to evaluate ICU admission rates. However, similar intubations rates were reported in a randomized controlled trial of infants with bronchiolitis who received CPAP via mask or helmet interfaces (17% vs. 23%, respectively; n = 30) [19].
In the present study, both groups tolerated the CPAP treatments equally well, and there were no differences in overall patient-reported comfort. Patient tolerance to treatment provided via mask or helmet interfaces has been compared in many studies [17, 18, 23,24,25,26,27,28,29,30]. Several studies have reported higher tolerance of the helmet interface [17, 23,24,25,26,27], whereas some studies reported no differences [21,22,23,24]. In our study, the helmet treatment was briefly paused in 26 cases; however, all patients completed the full 6 h treatment. All patients given ICPAP tolerated the treatment without breaks. Patients given HCPAP were more likely to report claustrophobia than those given ICPAP; however, no pauses in HCPAP treatment were due to claustrophobia.
The helmet interface reportedly reduces the risks of severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) aerosolization and exposure for healthcare personnel significantly, compared with the face mask interface [31]. Therefore, international guidelines have recommended using the helmet interface for noninvasive respiratory support during the SARS‑CoV‑2 pandemic [31]. Our results indicate that the helmet interface is as tolerated by patients and provides similar pulmonary oxygenation levels to the mask interface without increasing the risk of PPCs. Hence, the helmet interface may represent a safer option for staff without compromising patient care. Although in our experience, the protocol to remove the helmet for hasty intubation can be cumbersome compared to removing the mask.
This study had some limitations. First, patients in the ICPAP group received the standard care regime of CPAP at 10 cm H2O for 10 min every 2 h throughout their stay in the PACU, whereas those in the HCPAP group received 7.5 cm H2O for 6 h, initiated upon PACU arrival. However, the difference in the total length of CPAP treatment time [mask: median, 70 min (range, 66–80 min); helmet: median, 358 min (range, 350–360 min)] did not translate into improved oxygenation or a reduced risk of pulmonary complications in the HCPAP group. Second, the CPAP level in the HCPAP group (7.5 cm) was lower than in the ICPAP group (10 cm), and this may have offset any benefit of the longer CPAP duration in the HCPAP group. This HCPAP level was chosen based on previous studies [12, 13], and the lower pressure was also assumed to increase patient compliance. Therefore, this study compared two different CPAP treatment strategies rather than a direct comparison of two different CPAP interfaces using the same pressure level and treatment duration, which should be evaluated in future studies. Third, postoperative pain and management during the PACU visit was not documented. It was assessed using NRS (0–10) and treated with epidural boluses or IV opioids. As postoperative pain has been shown to reduce effort-dependent lung function and could influence the risk of PPC [3], registration of NRS scores and pain management during PACU admission would have been relevant and strengthened the study In addition, only limited blinding of treatment allocation was possible in this study, increasing the risk of bias. However, the investigator conducting the statistical analysis was blinded to treatment allocation. Furthermore, our results may lack external validity as this was a single-center study.
We found that continuous prophylactic CPAP treatment via the helmet interface was well tolerated by patients, provided a similar level of postoperative pulmonary oxygenation, and did not increase the risk of pulmonary complications compared to ICPAP. The helmet interface is associated with significantly lower risks of SARS-CoV-2 aerosolization and exposure for healthcare personnel [31], hence it may contribute to a safer PACU environment for staff without compromising treatment outcomes for patients recovering from major abdominal surgery.
References
Abbott TEF, Fowler AJ, Pelosi P, et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth. 2018;120(5):1066–79. https://doi.org/10.1016/j.bja.2018.02.007.
Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measur. Eur J Anaesthesiol. 2015;32(2):88–105. https://doi.org/10.1097/EJA.0000000000000118.
Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth. 2017;118(3):317–34. https://doi.org/10.1093/bja/aex002.
Ferreyra GP, Baussano I, Squadrone V, et al. Continuous positive airway pressure for treatment of respiratory complications after abdominal surgery: a systematic review and meta-analysis. Ann Surg. 2008;247(4):617–26. https://doi.org/10.1097/SLA.0b013e3181675829.
Ireland CJ, Chapman TM, Mathew SF, Herbison GP, Zacharias M. Continuous positive airway pressure (CPAP) during the postoperative period for prevention of postoperative morbidity and mortality following major abdominal surgery. Cochrane Database Syst Rev. 2014. https://doi.org/10.1002/14651858.CD008930.pub2.
Chiumello D, Chevallard G, Gregoretti C. Non-invasive ventilation in postoperative patients: a systematic review. Intensive Care Med. 2011;37(6):918–29. https://doi.org/10.1007/s00134-011-2210-8.
Lindner KH, Lotz P, Ahnefeld FW. Continuous positive airway pressure effect on functional residual capacity, vital capacity and its subdivisions. Chest. 1987;92(1):66–70. https://doi.org/10.1378/chest.92.1.66.
Pelosi P, Jaber S. Noninvasive respiratory support in the perioperative period. Curr Opin Anaesthesiol. 2010;23(2):233–8. https://doi.org/10.1097/ACO.0b013e328335daec.
Crimi C, Noto A, Princi P, Esquinas A, Nava S. A European survey of noninvasive ventilation practices. Eur Respir J. 2010;36(2):362–9. https://doi.org/10.1183/09031936.00123509.
Esquinas Rodriguez AM, Papadakos PJ, Carron M, Cosentini R, Chiumello D. Clinical review: helmet and non-invasive mechanical ventilation in critically ill patients. Crit Care. 2013;17(2):223. https://doi.org/10.1186/cc11875.
Canet J, Paluzie G, Valle J, Castillo J, Ph D, Sabate S. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113(6):1338–50.
Squadrone V, Coha M, Cerutti E, et al. CPAP for treatment of postoperative hypoxemia. JAMA. 2005;293(5):589–95.
Stock MC, Downs JB, Gauer PK, Alster JM, Imrey PB. Prevention of postoperative pulmonary complications with CPAP, incentive spirometry, and conservative therapy. Chest. 1985;87(2):151–7. https://doi.org/10.1378/chest.87.2.151.
Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg. 1970;49(6):924–34. https://doi.org/10.1213/00000539-197011000-00020.
Barbagallo M, Ortu A, Spadini E, et al. Prophylactic use of helmet CPAP after pulmonary lobectomy: a prospective randomized controlled study. Respir Care. 2012;57(9):1418–24. https://doi.org/10.4187/respcare.00838.
Pearse R, Ranieri M, Abbott T, et al. Postoperative continuous positive airway pressure to prevent pneumonia, re-intubation, and death after major abdominal surgery (PRISM): a multicentre, open-label, randomised, phase 3 trial. Lancet Respir Med. 2021;9(11):1221–30. https://doi.org/10.1016/s2213-2600(21)00089-8.
Principi T, Pantanetti S, Catani F, et al. Noninvasive continuous positive airway pressure delivered by helmet in hematological malignancy patients with hypoxemic acute respiratory failure. Intensive Care Med. 2004;30(1):147–50. https://doi.org/10.1007/s00134-003-2056-9.
Tonnelier JM, Prat G, Nowak E, et al. Noninvasive continuous positive airway pressure ventilation using a new helmet interface: a case–control prospective pilot study. Intensive Care Med. 2003;29(11):2077–80. https://doi.org/10.1007/s00134-003-1925-6.
Chidini G, Piastra M, Marchesi T, et al. Continuous positive airway pressure with helmet versus mask in infants with bronchiolitis: an RCT. Pediatrics. 2015;135(4):e868–75. https://doi.org/10.1542/peds.2014-1142.
Patroniti N, Foti G, Manfio A, Coppo A, Bellani G, Pesenti A. Head helmet versus face mask for non-invasive continuous positive airway pressure: a physiological study. Intensive Care Med. 2003;29(10):1680–7. https://doi.org/10.1007/s00134-003-1931-8.
Taccone P, Hess D, Caironi P, Bigatello LM. Continuous positive airway pressure delivered with a “helmet”: effects on carbon dioxide rebreathing*. Crtic Care Med. 2004. https://doi.org/10.1097/01.CCM.0000142577.63316.C0.
Ricksten SE, Bengtsson A, Soderberg C, Thorden M, Kvist H. Effects of periodic positive airway pressure by mask on postoperative pulmonary function. Chest. 1986;89(6):774–81. https://doi.org/10.1378/chest.89.6.774.
Rocco M, Dell’Utri D, Morelli A, et al. Noninvasive ventilation by helmet or face mask in immunocompromised patients: a case-control study. Chest. 2004;126(5):1508–15. https://doi.org/10.1378/chest.126.5.1508.
Conti G, Cavaliere F, Costa R, et al. Noninvasive positive-pressure ventilation with different interfaces in patients with respiratory failure after abdominal surgery: a matched-control study. Respir Care. 2007;52(11):1463–71.
Antonelli M, Conti G, Pelosi P, et al. New treatment of acute hypoxemic respiratory failure: noninvasive pressure support ventilation delivered by helmet—a pilot controlled trial. Crit Care Med. 2002;30(3):602–8. https://doi.org/10.1097/00003246-200203000-00019.
Antonelli M, Pennisi MA, Pelosi P, et al. Noninvasive positive pressure ventilation using a helmet in patients with acute exacerbation of chronic obstructive pulmonary disease: a feasibility study. Anesthesiology. 2004;100(1):16–24. https://doi.org/10.1097/00000542-200401000-00007.
Antonaglia V, Ferluga M, Molino R, et al. Comparison of noninvasive ventilation by sequential use of mask and helmet versus mask in acute exacerbation of chronic obstructive pulmonary disease: a preliminary study. Respiration. 2011;82(2):148–54. https://doi.org/10.1159/000324259.
Navalesi P, Costa R, Ceriana P, et al. Non-invasive ventilation in chronic obstructive pulmonary disease patients: helmet versus facial mask. Intensive Care Med. 2007;33(1):74–81. https://doi.org/10.1007/s00134-006-0391-3.
Vargas F, Thille A, Lyazidi A, Master BE, Campo FR, Brochard L. Helmet with specific settings versus facemask for noninvasive ventilation. Crit Care Med. 2009;37(6):1921–8. https://doi.org/10.1097/CCM.0b013e31819fff93.
Rocco M, Conti G, Alessandri E, et al. Rescue treatment for noninvasive ventilation failure due to interface intolerance with remifentanil analgosedation: a pilot study. Intensive Care Med. 2010;36(12):2060–5. https://doi.org/10.1007/s00134-010-2026-y.
Amirfarzan H, Cereda M, Gaulton TG, et al. Use of helmet CPAP in COVID-19—a practical review. Pulmonology. 2021. https://doi.org/10.1016/j.pulmoe.2021.01.008.
Funding
The study received funding for equipment purchase from “Læge Sofus Carl Emil Friis og Hustru Olga Doris Friis’ Legat”, no grant number was provided.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation and data collection was performed by JO, RBS, LH, KM, TT, CRM and ØJ. Data analysis and interpretation was performed by JO, RBS, MPA and ØJ. The first draft of the manuscript was written by JO. All authors read, performed critical revision, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or non-financial interest to disclose.
Ethical approval
This single-center single-blinded randomized controlled trial was approved by the Regional ethical committees of the capital region of Denmark (H-1-2013-094) and registered at clinicaltials.gov (NCT02173327).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Osterkamp, J.T.F., Strandby, R.B., Henningsen, L. et al. Comparing the effects of continuous positive airway pressure via mask or helmet interface on oxygenation and pulmonary complications after major abdominal surgery: a randomized trial. J Clin Monit Comput 37, 63–70 (2023). https://doi.org/10.1007/s10877-022-00857-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-022-00857-7