Abstract
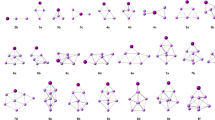
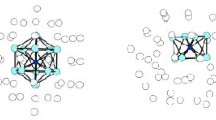
We have studied the structure and stability of the ionic Li+Xen and neutral LiXen (n = 1–35) small clusters. The potential energy surface of the ionic cluster is described using additive potentials, which represent the pair interactions taken from the best available coupled cluster ab initio calculations. The V +Li Xe and VXe−Xe potentials have been fitted by Tang and Toennies and Lennard-Jones (LJ) forms, respectively. The structure of LiXen neutral clusters have been investigated using a model potential and ab initio calculations. We have used the Li+Xe potential in its ground state and fitted to the Tang and Toennies formula. The LiXen optimized geometry is, then, used for one electron self consistent filed calculation of the only alkali valence electron interacting with the Li+Xen cluster. In order to determine the geometry of Li+Xen and LiXen clusters and their isomers, the potential energy surface has been explored by the Monte Carlo basin Hopping method. Their relative stability was studied by evaluating the energy and the energy differences as function of number n of Xenon atoms in clusters. It was shown, for Li+Xen, that n = 4, 6, 10, 14, 16, 18, 20, 22, 24, 26, 28 and 30 are the most stable structures.






Similar content being viewed by others
References
C. Tsoo, D. Estrin, and S. Singer (1990). J. Chem. Phys. 93, 7187.
J. Maclyn, M. McCarty, and G. W. Robinson (1959). Mol. Phys. 2, 415.
B. Meyer (1965). J. Chem. Phys. 43, 2986.
L. C. Balling, M. D. Havey, and J. Dawson (1978). F. J. Chem. Phys. 69, 1670.
L. C. Balling, J. F. Dawson, and M. D. Havey (1979). J. Phys. Rev. Lett. 43, 435.
J. J. Wright and L. C. Balling (1978). J. Chem. Phys. 73, 994.
L. C. Balling and J. J. Wright (1983). J. Chem. Phys. 79, 2941.
L. C. Balling and J. J. Wright (1984). J. Chem. Phys. 81, 675.
J. A. Boatz and M. E. Fajardo (1994). J. Chem. Phys. 101, 3472.
G. Martyna, C. Cheng, and M. L. Klein (1991). J. Chem. Phys. 95, 1318.
George E. Froudakis and Stavros C. Farantos (2000). M. Velegrakis. J. Chem. Phys. 258, 13.
Dhiflaoui J, Bouzouita H and Berriche H, Computation in Modern Science and Engineering, Proceeding of the International Conference on Computational Methods in Science and Engineering 2007, edited by T. E. Simos and G. Maroulis, American Institute of Physics. 2 CP 963, (2007).
D. Prekas, C. Lüder, and M. Velegrakis (1998). J. Chem. Phys. 108, 4450.
G. S. Fanourgakis, S. C. Farantos, C. Lüder, M. Velegrakis, and S. S. Xantheas (1996). J. Chem. Phys. 109, 108.
M. Ben El Hadj Rhouma, H. Berriche, Z. Ben Lakhdar, and F. Spiegelman (2004). Int J. Quant. Chem. 99, 495.
C. Lüder, D. Prekas, and M. Velegrakis (1997). Laser Chem. 17, 109.
M. El Hadj Rhouma, H. Berriche, Z. Ben Lakdhar, and F. Spiegelman (2002). J. Chem. Phys. 116, 1839.
M. El Hadj Rhouma, Z. Ben Lakdhar, H. Berriche, and F. Spiegelman (2006). J. Chem. Phys. 125, 084315.
Akihiro Fujisakia (1995). J. Chem. Phys. 102, 8485.
J. Dhiflaoui, H. Bouzouita, and H. Berriche (2009). Phys. Procedia 2, 1175.
K. T. Tang and J. P. Toennies (1984). J. Chem. Phys. 80, 3726.
J. Lozeille, E. Winata, P. Soldán, E. P. F. Lee, L. A. Viehland, and T. G. Wright (2002). Phys. Chem. Chem. Phys. 4, 3601.
E. A. Mason and H. W. Schamp (1958). Ann. Phys.(N.Y) 4, 233.
D. J. Wales and J. K. P. Doye (1997). J. Phys. Chem. A. 101, 5111.
J. E. Jones and A. E. Ingham (1925). Proc. R. Soc. A. 107, 636.
Acknowledgments
This work has been supported by the Advanced Materials Center and KACST through the Long-Term Comprehensive National Plan for Science, Technology and Innovation Program (Project no. 10-ADV1164-07).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Ahmari, M., Saidi, S., Dhiflaoui, J. et al. Structure and Stability of the Li+Xen and LiXen Clusters. J Clust Sci 26, 913–924 (2015). https://doi.org/10.1007/s10876-014-0780-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-014-0780-7