Abstract
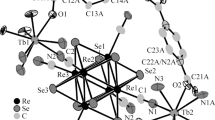
Two novel cluster organic frameworks derived from pyridine-2,6-dicarboxylate (PDA) and oxalate (ox2−) have been hydrothermally made: [Eu3(SO4)(PDA)3(ox)0.5(H2O)5]·4H2O (1) and Er(PDA)(ox)0.5(H2O) (2). Compound 1 possesses one-dimensional chain structure constructed from the alternate linkage of tetranuclear [Eu4(SO4)2]8+ (Eu4) and dinuclear [Eu2(ox)]4+ (Eu2) clusters. Compound 2 is a two-dimensional layer based on dimeric [Er2(COO)2]2+ (Er2) cluster units. Interestingly, such layer can be intuitively viewed as the linkages of helical chains and oxalate. In these two compounds, all anions are bivalent, and the ratio of trivalent lanthanide ions to these dianions is 2:3. Furthermore, compound 1 exhibits strong red luminescence upon 276 nm excitation.








Similar content being viewed by others
References
R. Sessoli and A. K. Powell (2009). Chem. Soc. Rev. 253, 2328.
A. Müller, E. Beckmann, H. Bögge, M. Schmidtmann, and A. Dress (2002). Nature 41, 1162.
A. J. Tasiopoulos, A. Vinslava, W. Wernsdorfer, K. A. Abboud, and G. Christou (2004). Angew. Chem. Int. Ed. 43, 2117.
M. B. Zhang, J. Zhang, S. T. Zheng, and G. Y. Yang (2005). Angew. Chem. Int. Ed. 44, 1385.
X. J. Kong, Y. L. Wu, L. S. Long, L. S. Zheng, and Z. P. Zheng (2009). J. Am. Chem. Soc. 131, 6918.
Z. P. Zheng (2001). Chem. Commun. 2521.
M. Wu, F. Jiang, X. Kong, D. Yuan, L. Long, S. A. AL-Thabaiti, and M. Hong (2013). Chem. Sci. 4, 3104.
G. Calvez, C. Daiguebonne, and O. Guillou (2011). Inorg. Chem. 50, 2851.
W. H. Fang, L. Cheng, L. Huang, and G. Y. Yang (2013). Inorg. Chem. 52, 6.
J. W. Cheng, J. Zhang, S. T. Zheng, M. B. Zhang, and G. Y. Yang (2006). Angew. Chem. Int. Ed. 45, 73.
J. W. Cheng, J. Zhang, S. T. Zheng, and G. Y. Yang (2008). Chem.-Eur. J. 14, 88.
W. H. Fang, J. W. Cheng, and G. Y. Yang (2014). Chem.-Eur. J. 20, 2704.
X. J. Gu and D. F. Xue (2007). Inorg. Chem. 46, 5349.
J. W. Cheng, S. T. Zheng, W. Liu, and G. Y. Yang (2008). CrystEngComm 10, 1047.
M. D. Allendorf, C. A. Bauer, R. K. Bhakta, and R. J. T. Houk (2009). Chem. Soc. Rev. 38, 1330.
W. X. Feng, Y. Zhang, Z. Zhang, X. Q. Lu, H. Liu, G. X. Shi, D. Zou, J. R. Song, D. D. Fan, W. K. Wong, and R. A. Jones (2012). Inorg. Chem. 51, 11377.
Y. Q. Sun, J. Zhang, Y. M. Chen, and G. Y. Yang (2005). Angew. Chem. Int. Ed. 44, 5814.
J. Xu, W. P. Su, and M. C. Hong (2011). Cryst. Growth Des. 11, 337.
G. Peng, L. Ma, L. Liang, Y. Ma, C. Yang, and H. Deng (2013). CrystEngComm 15, 922.
B. Zhao, L. Yi, P. Cheng, D. Z. Liao, S. P. Yan, and Z. H. Jiang (2004). Inorg. Chem. Commun. 7, 971.
H. Eshtiagh-Hosseini, H. Aghabozorg, M. Mirzaei, M. M. Amini, Y. G. Chen, A. Shokrollahi, and R. Aghaei (2010). J. Mol. Struct. 973, 180.
M. Frisch, and C. L. Cahill (2006). Dalton Trans. 4679.
D. Banerjee, S. J. Kim, L. A. Borkowski, W. Xu, and J. B. Parise (2009). Cryst. Growth Des. 10, 709.
X. J. Kong, Y. P. Ren, L. S. Long, Z. P. Zheng, G. Nichol, R. B. Huang, and L. S. Zheng (2008). Inorg. Chem. 47, 2728.
X. Feng, J. S. Zhao, L. Y. Wang, and X. G. Shi (2009). Inorg. Chem. Commun. 12, 388.
X. Feng, B. Liu, L. Y. Wang, J. S. Zhao, J. G. Wang, N. S. Weng, and X. G. Shi (2010). Dalton Trans. 39, 8038.
X. Feng, L. Y. Wang, J. S. Zhao, J. G. Wang, N. S. Weng, B. Liu, and X. G. Shi (2010). CrystEngComm 12, 774.
M. S. Liu, Q. Y. Yu, Y. P. Cai, C. Y. Su, X. M. Lin, X. X. Zhou, and J. W. Cai (2008). Cryst. Growth Des. 8, 4083.
G. M. Sheldrick SADABS, Program for Siemens Area Detector Absorption Corrections (University of Göttingen, Göttingen, Germany, 1997).
G. M. Sheldrick SHELXL97, Program for Crystal Structure Refinement (University of Göttingen, Göttingen, Germany, 1997).
G. M. Sheldrick SHELXS97, Program for Crystal Structure Solution (University of Göttingen, Göttingen, Germany, 1997).
X. Y. Chen, X. P. Yang, and B. J. Holliday (2010). Inorg. Chem. 49, 2538.
J. Cepeda, R. Balda, G. Beobide, O. Castillo, J. Fernandez, A. Luque, S. Perez-Yanez, P. Roman, and D. Vallejo-Sanchez (2011). Inorg. Chem. 50, 8437.
B. Q. Ma, D. S. Zhang, S. Gao, T. Z. Jin, C. H. Yan, and G. X. Xu (2000). Angew. Chem. Int. Ed. 39, 3644.
W. H. Wang, H. R. Tian, Z. C. Zhou, Y. L. Feng, and J. W. Cheng (2012). Cryst. Growth Des. 12, 2567.
G. Abbas, Y. H. Lan, G. E. Kostakis, W. Wernsdorfer, C. E. Anson, and A. K. Powell (2010). Inorg. Chem. 49, 8067.
D. M. M. Freckmann, T. Dube, C. D. Berube, S. Gambarotta, and G. P. A. Yap (2002). Organometallics 21, 1240.
I. A. Gass, B. Moubaraki, S. K. Langley, S. R. Batten, and K. S. Murray (2012). Chem. Commun. 48, 2089.
W. H. Fang and G. Y. Yang (2014). J. Cluster Sci. doi:10.1007/s10876-014-0717-1.
Y. Q. Sun, J. Zhang, and G. Y. Yang (2006). Chem. Commun. 1947.
A. F. Kirby, D. Foster, and F. S. Richardson (1983). Chem. Phys. Lett. 95, 507.
Acknowledgments
This work was supported by the NSFC (Nos. 91122028, 21221001, and 50872133), the 973 Program (Nos. 2014CB932101 and 2011CB932504), the NSFC for Distinguished Young Scholars (No. 20725101).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fang, WH., Jia, XL. & Yang, GY. Lanthanide Cluster Organic Frameworks Derived from Pyridine-2,6-dicarboxylate and Oxalate: Syntheses, Structures and Luminescence. J Clust Sci 25, 1553–1565 (2014). https://doi.org/10.1007/s10876-014-0751-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-014-0751-z