Abstract
The role of class IA phosphoinositide 3 kinases (PI3Ks) in immune function and regulation continues to expand with the identification of greater numbers of genetic variants. This case report is the second reported case of a homozygous premature stop codon within the PIK3R1 gene leading to autosomal recessive agammaglobulinemia. The proband, born to consanguineous parents, presented at 10 months of age with a history of oropharyngeal petechiae and bleeding from the mouth, gums, and tear ducts. Initial investigations revealed thrombocytopenia, neutropenia and the absence of B cells. Further genetic testing via a custom next-generation sequencing panel confirmed the presence of a homozygous mutation in PIK3R1, c.901 C>T, a premature stop codon at amino acid position 301. Given their many roles in immune regulation, recessive mutations in the PlK3R1 gene should be considered in infants presenting with hypogammaglobulinemia or agammaglobulinemia, particularly in the setting of parental consanguinity.


Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Conley ME, Mathias D, Treadaway J, Minegishi Y, Rohrer J. Mutations in Btk in patients with presumed X-linked agammaglobulinemia. Am J Hum Genet. 1998;62(5):1034–43. https://doi.org/10.1086/301828.
Conley ME, Dobbs AK, Farmer DM, Kilic S, Paris K, Grigoriadou S, et al. Primary B cell immunodeficiencies: comparisons and contrasts. Annu Rev Immunol. 2009;27(1):199–227. https://doi.org/10.1146/annurev.immunol.021908.132649.
Whitman M, Kaplan DR, Schaffhausen B, Cantley L, Roberts TM. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985;315(6016):239–42. https://doi.org/10.1038/315239a0.
Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3(4):317–30. https://doi.org/10.1038/nri1056.
Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–19. https://doi.org/10.1038/nrg1879.
Okkenhaug K, Ali K, Vanhaesebroeck B. Antigen receptor signalling: a distinctive role for the p110δ isoform of PI3K. Trends Immunol. 2007;28(2):80–7. https://doi.org/10.1016/j.it.2006.12.007.
Inukai K, Funaki M, Ogihara T, Katagiri H, Kanda A, Anai M, et al. p85alpha gene generates three isoforms of regulatory subunit for phosphatidylinositol 3-kinase (PI 3-Kinase), p50alpha, p55alpha, and p85alpha, with different PI 3-kinase activity elevating responses to insulin. J Biol Chem. 1997;272(12):7873–7882. http://www.ncbi.nlm.nih.gov/pubmed/9065454. https://doi.org/10.1074/jbc.272.12.7873.
Fruman DA. Regulatory subunits of class IA PI3K. In: Rommel C, Vanhaesebroeck B, Vogt PK, editors. Phosphoinositide 3-Kinase Heal. Dis, vol. 1. Berlin Heidelberg: Springer-Verlag; 2010. p. 225–44. https://doi.org/10.1007/82_2010_39.
Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11(5):329–41. https://doi.org/10.1038/nrm2882.
Deane JA, Kharas MG, Oak JS, Stiles LN, Luo J, Moore TI, et al. T-cell function is partially maintained in the absence of class IA phosphoinositide 3-kinase signaling. Blood. 2007;109(7):2894–902. https://doi.org/10.1182/blood-2006-07-038620.
Okkenhaug K, Fruman DA. PI3Ks in lymphocyte signaling and development. In: Rommel C, Vanhaesebroeck B, Vogt PK, editors. Phosphoinositide 3-Kinase Heal. Dis, vol. 1. Berlin Heidelberg: Springer-Verlag; 2010. p. 57–85. https://doi.org/10.1007/82_2010_45.
Conley ME, Dobbs AK, Quintana AM, Bosompem A, Wang Y-D, Coustan-Smith E, et al. Agammaglobulinemia and absent B lineage cells in a patient lacking the p85α subunit of PI3K. J Exp Med. 2012;209(3):463–70. https://doi.org/10.1084/jem.20112533.
Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, et al. Clinical trials group, lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112(5):973–80. https://doi.org/10.1016/j.jaci.2003.07.003.
de la Morena M, Haire RN, Ohta Y, Nelson RP, Litman RT, Day NK, et al. Predominance of sterile immunoglobulin transcripts in a female phenotypically resembling Bruton’s agammaglobulinemia. Eur J Immunol. 1995;25(3):809–15. https://doi.org/10.1002/eji.1830250327.
Sheen J-M, Kuo H-C, Yu H-R, Huang E-Y, Wu C-C, Yang KD. Prolonged acquired neutropenia in children. Pediatr Blood Cancer. 2009;53(7):1284–8. https://doi.org/10.1002/pbc.22247.
Elkaim E, Neven B, Bruneau J, Mitsui-Sekinaka K, Stanislas A, Heurtier L, et al. Clinical and immunologic phenotype associated with activated phosphoinositide 3-kinase δ syndrome 2: a cohort study. J Allergy Clin Immunol. 2016;138(1):210–218.e9. https://doi.org/10.1016/j.jaci.2016.03.022.
Lougaris V, Faletra F, Lanzi G, Vozzi D, Marcuzzi A, Valencic E, et al. Altered germinal center reaction and abnormal B cell peripheral maturation in PI3KR1-mutated patients presenting with HIGM-like phenotype. Clin Immunol. 2015;159(1):33–6. https://doi.org/10.1016/j.clim.2015.04.014.
Deau M-C, Heurtier L, Frange P, Suarez F, Bole-Feysot C, Nitschke P, et al. A human immunodeficiency caused by mutations in the PIK3R1 gene. J Clin Invest. 2014;124(9):3923–8. https://doi.org/10.1172/JCI75746.
Deau M-C, Heurtier L, Frange P, Suarez F, Bole-Feysot C, Nitschke P, et al. A human immunodeficiency caused by mutations in the PIK3R1 gene. J Clin Invest. 2015;125(4):1764–5. https://doi.org/10.1172/JCI81746.
Lucas CL, Zhang Y, Venida A, Wang Y, Hughes J, McElwee J, et al. Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K. J Exp Med. 2014;211(13):2537–47. https://doi.org/10.1084/jem.20141759.
Watanabe N, Nakajima H, Suzuki H, Oda A, Matsubara Y, Moroi M, et al. Functional phenotype of phosphoinositide 3-kinase p85alpha-null platelets characterized by an impaired response to GP VI stimulation. Blood. 2003;102(2):541–8. https://doi.org/10.1182/blood-2002-11-3327.
Walsh G, Flower CD, Nasser S, Ewan PW. Granulomatous interstitial pneumonitis in association with primary hypogammaglobulinemia: computed tomography appearances. J Thorac Imaging. 1999;14:207–209. http://www.ncbi.nlm.nih.gov/pubmed/10404507. https://doi.org/10.1097/00005382-199907000-00008.
Walsh CM, Fruman DA. Too much of a good thing: immunodeficiency due to hyperactive PI3K signaling. J Clin Invest. 2014;124(9):3688–90. https://doi.org/10.1172/JCI77198.
Jaiswal BS, Janakiraman V, Kljavin NM, Chaudhuri S, Stern HM, Wang W, et al. Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer Cell. 2009;16(6):463–74. https://doi.org/10.1016/j.ccr.2009.10.016.
Mandelker D, Gabelli SB, Schmidt-Kittler O, Zhu J, Cheong I, Huang C-H, et al. A frequent kinase domain mutation that changes the interaction between PI3Kalpha and the membrane. Proc Natl Acad Sci U S A. 2009;106(40):16996–7001. https://doi.org/10.1073/pnas.0908444106.
Sun M, Hillmann P, Hofmann BT, Hart JR, Vogt PK. Cancer-derived mutations in the regulatory subunit p85alpha of phosphoinositide 3-kinase function through the catalytic subunit p110alpha. Proc Natl Acad Sci U S A. 2010;107(35):15547–52. https://doi.org/10.1073/pnas.1009652107.
Parsons DW, Jones S, Zhang X, Lin JC-H, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–12. https://doi.org/10.1126/science.1164382.
Kracker S, Curtis J, Ibrahim MAA, Sediva A, Salisbury J, Campr V, et al. Occurrence of B-cell lymphomas in patients with activated phosphoinositide 3-kinase δ syndrome. J Allergy Clin Immunol. 2014;134(1):233–6. https://doi.org/10.1016/j.jaci.2014.02.020.
Zhang J, Grubor V, Love CL, Banerjee A, Richards KL, Mieczkowski PA, et al. Genetic heterogeneity of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2013;110(4):1398–403. https://doi.org/10.1073/pnas.1205299110.
Crank MC, Grossman JK, Moir S, Pittaluga S, Buckner CM, Kardava L, et al. Mutations in PIK3CD can cause hyper IgM syndrome (HIGM) associated with increased cancer susceptibility. J Clin Immunol. 2014;34(3):272–6. https://doi.org/10.1007/s10875-014-0012-9.
Petrovski S, Parrott RE, Roberts JL, Huang H, Yang J, Gorentla B, et al. Dominant splice site mutations in PIK3R1 cause hyper IgM syndrome, lymphadenopathy and short stature. J Clin Immunol. 2016;36(5):462–71. https://doi.org/10.1007/s10875-016-0281-6.
Dyment DA, Smith AC, Alcantara D, Schwartzentruber JA, Basel-Vanagaite L, Curry CJ, et al. Mutations in PIK3R1 cause SHORT syndrome. Am J Hum Genet. 2013;93(1):158–66. https://doi.org/10.1016/j.ajhg.2013.06.005.
Acknowledgements
The authors would like to thank the family for generously providing their permission to be included in this publication, as well as all those who contributed to the care of this patient and their sibling. Special acknowledgements to Dr. Victoria Siu for her contributions and support.
Authorship Statement
Manuscript title: A novel case of autosomal recessive agammaglobulinemia due to a homozygous mutation in PIK3R1.
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Furthermore, each author certifies that this material or similar material has not been and will not be submitted to or published in any other publication before its appearance in the Journal of Clinical Immunology.
Authorship Contributions
Category 1
Conception and design of study: Paoyun Tang
Acquisition of data: Paoyun Tang, Sharan Goobie, Meghan Clynick, Julia Upton
Analysis and/or interpretation of data: Paoyun Tang, Sharan Goobie, Julia Upton
Category 2
Drafting the manuscript: Paoyun Tang, Meghan Clynick
Revising the manuscript critically for important intellectual content: Julia Upton, Sharan Goobie, Michelle Barton-Forbes, Marina Salvadori, April Price
Category 3
Approval of the version of the manuscript to be published: Paoyun Tang, Sharan Goobie, Meghan Clynick, Julia Upton, Michelle Barton-Forbes, Marina Salvadori, April Price
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent from the family for inclusion in this report was obtained with a translator present.
Conflict of Interest
JU is a member of the Medical Advisory Board for Immunodeficiency Canada and her institution received research support from Kedrion. The remaining authors declare that they have no conflicts of interest.
Additional information
Statement of Novelty
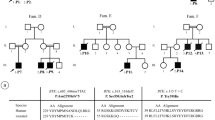
This case report identifies a novel recessive mutation in exon 7 of the PIK3R1 gene in a female with autosomal recessive agammaglobulinemia.
Rights and permissions
About this article
Cite this article
Tang, P., Upton, J.E.M., Barton-Forbes, M.A. et al. Autosomal Recessive Agammaglobulinemia Due to a Homozygous Mutation in PIK3R1 . J Clin Immunol 38, 88–95 (2018). https://doi.org/10.1007/s10875-017-0462-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-017-0462-y