Abstract
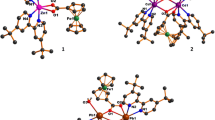
The centrosymmetric dimer zinc compound, [Zn(HL)(bpy)2(H2O)]2(L), (L = O2C(CF2)6CO2, bpy = 2,2′-bipyridine), was obtained through the reaction of Zn(ClO4)2·6H2O, bpy and perfluorosuberic acid. Zn(II) centre is coordinated by four N atoms from two bpy ligands and two O atoms from a water molecule and monoanionic suberate ligand in a distorted octahedral coordination environment. The unit structure contains crystallographically centrosymmetric suberate anion which acts as a bidentate bridging ligand between each cationic monomer complex unit via hydrogen bonding. The very strong interaction of hydrogen bonding of hydoxycarbonyl–carboxylate system in solid state has O–H···O 2.436(3) Å (O···H = 1.25(6) Å and H···O = 1.19(6) Å). These units are also connected to each other via π···π, C–H···π, C–F···π and F···F stacking interactions, C–H···O, O–H···O and C–H···F hydrogen bonds giving rise to a multi-dimensional network. The complex is the first reported example of a coordination compound based on both bpy ligands together with perfluorosuberic acid. Moreover, compound exhibit intense solid state fluorescent emissions at room temperature.
Graphical Abstract
The synthesis and X-ray characterization of unusual centrosymmetric dimer zinc compound, [Zn(HL)(bpy)2(H2O)]2(L) (L = O2C(CF2)6CO2, bpy = 2,2′bipyridine), and its photoluminescence property have been reported. The very strong interaction of hydrogen bonding of hydroxycarbonyl-carboxylate system in solid state has O–H···O 2.436(4) Å (O···H = 1.25 (7) Å, H···O = 1.20(7) Å and OHO = 168.4°).







Similar content being viewed by others
References
Gao J, Wang J, Nie J (2011) Acta Crystallogr C67:m181
Wang J, Tao JQ, Xu XJ (2011) Acta Crystallogr C67:m173
Kitagawa S, Uemura K (2005) Chem Soc Rev 34:109
Phan A, Doonan CJ, Uribe-Romo FJ, Knobler CB, O’Keeffe M, Yaghi OM (2010) Acc Chem Res 43:58
Yaghi OM, Li HL, Davis C, Richardson D, Groy TL (1998) Acc Chem Res 31:474
Jenniefer SJ, Muthiah PT (2011) Acta Crystallogr C67:m69
Xu G, Xie Y (2010) Acta Crystallogr C66:m201
Seo J, Matsuda R, Sakamoto H, Bonneau C, Kitagawa S (2009) J Am Chem Soc 131:12792
Kerbellec N, Kustaryono D, Haquin V, Etienne M, Daiguebonne C, Guillou O (2009) Inorg Chem 48:2837
Kani I, Büyükgüngör O, Şişman F (2006) Z Nat B 61b:1198
Kani I, Şahin O, Yılmaz F, Büyükgüngör O (2006) Acta Cryst E62:m1909
Kani I, Darak C, Şahin O, Büyükgüngör O (2008) Polyhedron 27:1238
Bruker APEX2 (Version 7.23A) and SAINT (Version 7.23A). Bruker AXS Inc., Madison (2007)
Sheldrick GM (2008) Acta Crystallogr A64:112
Sheldrick GM (1997) SHELXL-97. Universitat Göttingen, Göttingen
Spek AL (2005) Platon-A multipurpose crystallographic tool. Utrecht University, Utrecht
Chun H, Dybtsev DN, Kim H, Kim K (2005) Chem Eur J 11:3521
Sun CY, Dong B, Lv Q, Zheng XJ (2011) Z Anorg Allg Chem 637:276
Ye BH, Xue F, Xue GQ, Ji LN, Mak TCW (1999) Polyhedron 18:1785
Gilli P, Gilli G (2010) J Mol Struct 972:2
Gilli P, Bertolasi V, Ferretti V, Gilli G (1994) Am Chem Soc 116:909
Kovalchukova OV, Kuz’mina NE, Zaitsev BE, Strashnova SB, Palkina KK (2002) Dokl Phys Chem 386:251
Price DJ, Fristsch S, Wood PT, Powell AK (2005) Acta Crystallogr E61:m1174
Steiner T, Saenger W (1992) Acta Cyrstallogr. B48:819
Steiner T, Saenger W (1993) J Am Chem Soc 114:10146
Jeffrey GA, Mitra J (1984) J Am Chem Soc 106:5546
Dunitz D, Taylor R (1997) Chem Eur J 3:89
Lee H, Knobler CB, Hawthorne MF (2000) Chem Commun 2485
Bianchi R, Forni A, Pilati T (2003) Chem Eur J 9:1631
Bernstein J, Davis RE, Shimoni L, Chang NL (1995) Angew Chem Int Ed Engl 34:1555
Rybalova TV, Yu Bagryanskaya I (2009) J Struct Chem 50:741
Ramasubbu N, Parthasarathy R, Murray-Rust P (1986) J Am Chem Soc 108:4308
Bondi AJ (1996) Phys Chem 68:441
Dautel OJ, Fourmijue (2000) J Org Chem 65:6479
Madjaci NNL, Desiraju GR, Bilton C, Howard JAK, Allen FH (2000) Acta Cyristallogr. B56:1063
Prasanna MD, Guru Row TN (2000) Cryst Eng 3:135
Choudhury R, Guru Row TN (2004) Cryst Growth Des 4:47
Geraghty M, McCann M, Devereux M, McKee V (1999) Inorg Chim Acta 293:160
Zheng SL, Yang JH, Yu XL, Chen XM, Wong WT (2004) Inorg Chem 43:830
Tao J, Shi JX, Tong ML, Zhang XX, Chen XM (2001) Inorg Chem 40:6328
Chen W, Wang JY, Chen C, Yue Q, Yuan HM, Chen JS, Wang SN (2003) Inorg Chem 42:944
Yersin H, Vogler A (eds) (1987) Photochemistry and photophysics of coordination compounds. Springer, Berlin
Yang EC, Zhao HK, Ding B, Wang XG, Zhao XJ (2007) Cryst Growth Des 7:2009
Zheng XY, Ye LQ, Wen YH (2011) J Mol Struct 987:132
Guo HD, Guo XM, Batten SR, Song JF, Song SY, Dang S, Zheng GL, Tang JK, Zhang HJ (2009) Cryst Growth Des 9:1394
Lu J, Zhao K, Fang QR, Xu JQ, Yu JH, Zhang X, Bie HY, Wang TG (2005) Cryst Growth Des 5:1091
Zhang J, Xie YR, Ye Q, Xiong RG, Xue Z, You XZ (2003) Eur J Inorg Chem 2572
Acknowledgments
The author is grateful to Anadolu University and the Medicinal Plants and Medicine research Centre of Anadolu University, Eskişehir, Turkey, for the use of X-ray Diffractometer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kani, İ. Unusual Very Strong O–H···O Hydrogen Bonding in Zinc Complex: Crystal Structure and Photoluminescence of [Zn(HL)(bpy)2(H2O)]2(L) (L = O2C(CF2)6CO2, bpy = 2,2′bipyridine). J Chem Crystallogr 42, 832–838 (2012). https://doi.org/10.1007/s10870-012-0321-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-012-0321-x