Abstract
The crystal structures of the title compounds consist of 1,4-dimethyl-1,4-diazabicyclo[2.2.2]octane-1,4-diium cation [C8H18N2]2+ and [H2PO4]− or [HSO4]− anions. Both crystal structures are monoclinic, the structure of the dihydrogen phosphate (I) is non-centrosymmetric (P21) with a = 6.4090(2) Å, b = 13.6920(5) Å, c = 7.6140(3) Å, β = 94.620(2)°, V = 665.97(4) Å3, Z = 2; whereas the unit cell of the hydrogen sulphate (II) is centrosymmetric (P21/c) with a = 13.8460(2) Å, b = 12.6610(2) Å, c = 8.0360(2) Å, β = 99.5800(12)°, V = 1389.10(5) Å3, Z = 4. Both the structures are formed by the different bonding patterns of the anions interlinked by strong and moderate O–H···O hydrogen bonds. While the structure of (I) consists of a two-dimensional network of the hydrogen bonded dihydrogen phosphates, the infinite chains of the hydrogen bonded hydrogen sulphates are the basic building unit of the structure (II). In addition to the dominant electrostatic interaction the divalent cations stabilize themselves in the structures by forming several C–H···O hydrogen bonds to the oxygen atoms of the anions. The IR spectra of both the compounds are strongly affected by the hydrogen bonds whose influence on OH stretching vibrations is analysed by means of the DFT calculations in the solid state.
Graphical Abstract
The crystal structures of the 1,4-dimethyl-1,4-diazabicyclo[2.2.2]octane-1,4-diium dihydrogen phosphate and hydrogen sulphate comprise strong and moderate O–H···O hydrogen bonds significantly influencing the high energy region of IR spectra.

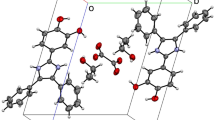
The crystal structure of the dihydrogen phosphate of the title cation viewed along the a axis







Similar content being viewed by others
References
Agmon N (1995) Chem Phys Lett 244:456
Kreuer KD (1996) Chem Mater 8:610
Baranov AI (2003) Crystallogr Rep 48:1012
Smrčok Ľ, Havlíček D, Kaman O, Rundlöf H (2009) Z Kristallogr 224:174
Chudoba V (2003) PhD thesis, Charles University in Prague, Czech Republic (In Czech)
Belushkin AV, Adams MA, Hull S, Shuvalov LA (1995) Solid State Ion 77:91
Chisholm CRI, Haile SM (1999) Acta Cryst B55:937
Wempe MF (2001) J Mol Struct 562:63
Otwinowski Z, Minor W (1997) HKL Denzo and Scalepack Program Package. Nonius BV, Delft
Blessing RH (1995) Acta Cryst A51:33
Altomare A, Burla MC, Camalli M, Cascarano G, Giacovazzo C, Guagliardi A, Polidori G (1994) J Appl Crystallogr 27:435
Sheldrick GM (1993) SHELX93, Program for crystal structure refinement. University of Gottingen, Germany
Spek AL (2002) PLATON. A multipurpose crystallographic tool. Utrecht University, Netherlands (www.cryst.ch-em.uu.nl/platon)
Brandenburg K (2006) Diamond. Version 3.1e. Crystal Impact GbR, Bonn, Germany
Kresse G, Hafner J (1993) Phys Rev B48:13115
Kresse G, Furthmüller J (1996) Phys Rev B54:11169
Kresse G, Furthmüller J (1996) Comp Mat Sci 6:15
Blöchl PE (1994) Phys Rev B50:17953
Kresse G, Joubert J (1999) Phys Rev B59:1758
Kresse G, Hafner J (1994) J Phys Condens Matter 6:8245
Teter MP, Payne MC, Allan DC (1989) Phys Rev B40:12255
Bylander DM, Kleinman L, Lee S (1990) Phys Rev B42:1394
Hafner J (2003) J Mol Struct 651–653:3
Portmann S, Luthi HP (2000) Chimia 54:766
Nosé SJ (1984) J Chem Phys 81:511
Ferrario M, Ryckaert JP (1985) Mol Phys 54:587
Ramirez-Cuesta AJ (2004) Comp Phys Commun 157:226
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, New York, p 222
Fergusson G, Glidewell C, Gregson RM, Meehan PR (1998) Acta Cryst B54:129
Amri M, Zouari N, Mhiri T, Pechev S, Gravereau P, Von Der Muhll R (2007) J Phys Chem Solids 68:1281
Hemamalini M, Muthiah PT, Rychlewska U, Plutecka A (2005) Acta Cryst C61:o95
Drozd M, Baran J (2006) Spectrochim Acta A 64:867
Sun XM, Qu Y (2005) Acta Cryst E61:m1360
Qu Y, Sun XM (2005) Acta Cryst E61:m2121
Wiebcke M, Emmer J, Felsche J, Hoebbel D, Engelhardt G (1994) Z Anorg Allg Chem 620:757
Janzen DE, Ewbank PC, Mann KR (2005) Acta Cryst C61:o631
Bratos S (1975) J Chem Phys 63:3499
Bratos S, Ratajczak H (1982) J Chem Phys 76:77
Fillaux F, Marchon B, Novak A (1984) Chem Phys 86:127
Fillaux F, Marchon B, Novak A (1989) Chem Phys 130:257
Koleva V, Stefov V, Cahil A, Najdoski M, Šoptrajanov B, Engelen B, Lutz HD (2009) J Mol Struct 919:164
Engelen B, Boldt K, Műller H, Unterderweide K (1997) J Mol Struct 436–437:247
Baran J, Trzebiatowska M, Ratajczak H (2004) J Mol Struct 708:127
Acknowledgments
Financial support by the Charles University in Prague, through the project GAUK 133508 and by the Long term research plan of the Ministry of Education of the Czech Republic through the project No. MSM 0021620857 is acknowledged. The work was partially supported by the Slovak Grant Agency VEGA under the contract No. 2/0150/09.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaman, O., Smrčok, Ľ., Císařová, I. et al. Dihydrogen Phosphate and Hydrogen Sulphate of 1,4-Dimethyl-1,4-diazabicyclo[2.2.2]octane-1,4-diium: Crystal Structures, Hydrogen Bonding and Infrared Spectra. J Chem Crystallogr 41, 1539–1546 (2011). https://doi.org/10.1007/s10870-011-0137-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-011-0137-0