Abstract
The title complex triaqua (3-hydroxy-5-hydroxymethyl-2-methylpyridine-4-carboxaldehyde-3-methylisothiosemicarbazone-k3,O3,N7,N10)Ni(II) nitrate ([Ni(PLITSC)(H2O)3](NO3)2, 1) represents the second transition metal complex incorporating an isothiosemicarbazide-pyridoxal based Schiff base that has been crystallographically characterized. Complex 1 crystallizes in a P21/n space group, with lattice constants: a = 11.2254(1) Å, b = 12.9941(2) Å, c = 12.8663(2), β = 96,7713(5)°, V = 1863.64(4) Å3, Z = 4, F(000) = 1016, R 1 = 0.0681, wR 2 = 0.1201. The central Ni(II) cation is found in a six-coordinate octahedral geometry formed by the tridentate Schiff base ligand PLITSC and three water molecules. The identity of 1 was further confirmed by elemental analysis, IR spectra, and conductometric and magnetochemical measurements.
Index Abstract
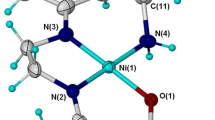
The coordination sphere of the Ni(II) cation in the green, paramagnetic complex [Ni(C10H14N4O2S1)(H2O)3](NO3)2 (1, Fig. 1), which crystallizes in P21/n space group, consists of three water molecules and a single tridentate ligand (Schiff base) obtained from isothiosemicarbazide and pyridoxal (pyridoxal is 3-hydroxy-5-hydroxymethyl-2-methylpyridine-4-carboxaldehyde) moieties.





Similar content being viewed by others
References
West DX, Padhye SB, Sonawane PB (1991) Structure and bonding, vol 76. Springer-Verlag, Berlin, pp 1–49
Casas JS, Garcia-Tasende MS, Sordo J (2000) Coord Chem Rev 209:197
West DX, Liberta AE, Padhye SB, Chikate RC, Sonawane PB, Kumbhar AS, Yeranade RG (1993) Coord Chem Rev 123:49
Belicchi Ferrari M, Bisceglie F, Casoli C, Durot S, Morgenstern-Badarau I, Pelosi G, Pilotti E, Tarasconi P (2005) J Med Chem 48:1671
Cardia MC, Begala M, Delogu A, Maccioni E, Plumitallo A (2000) Farmaco 55:93
Leovac V, Jevtovic V, Jovanovic Lj, Bogdanovic G (2005) J Serb Chem Soc 70:393
Jevtovic V (2002) Ph.D. Thesis, Faculty of Science, University of Novi Sad
Jevtovic V, Jovanovic Lj, Leovac V, Bjelica L (2003) J Serb Chem Soc 68:929
Lj Jovanovic, Jevtovic V, Leovac V, Bjelica L (2003) J Serb Chem Soc 70:187
Leovac V, Jevtovic V, Bogdanoviv G (2002) Acta Cryst C58:m514
Novakovic S, Tomic Z, Jevtovic V, Leovac V (2002) Acta Cryst C58:m358
Otwinowski Z, Minor W (1996) In: Carter CW, Sweet RM (eds) Methods enzymology. Academic Press, New York, USA
Nonius (1997) Delft BV, The Netherlands
Altomare A, Cascarano G, Giacovazzo C, Guagliardi A, Burla MC, Polidori G, Camalli M (1994) SIR 92-a program for automatic solution of crystal structures by direct methods. J Appl Crystallogr 27:435
Betteridge PW, Carruthers JR, Cooper RI, Prout J, Watkin DJ (2003) CRYSTAL version 12: software for guided crystal structure analysis. J Appl Crystallogr 36:1487
Belicchi-Ferrari M, Gasspari-Fava G, Pelizzi C, Pelosi G, Tarasconi P (1998) Inorg Chim Acta 269:297
Casas JS, Rodriquez-Arquelles MC, Russo U, Sanchez A, Sordo J, Vazquez-Lopez A, Pinelli S, Lunghi P, Bonati A, Albertini R (1998) J Inorg Chem 69:283
Belicchi-Ferrari M, Gasspari-Fava G, Leporati E, Pelizzi G, Tarasconi P, Tosi G (1986) J Chem Soc Dalton Trans 2455
Mohan M, Madhuranath PH, Kumar A, Kumar M, Jha NK (1989) Inorg Chem 28:96
Geary WJ (1971) Coord Chem Rev 12:73
Cotton FA, Wilkinson G (1988) Advanced inorganic chemistry. Wiley, New York
Acknowledgments
The author acknowledges the Oxford Chemical Crystallography Service for the use of the instrumentation and many thanks to the D.J. Watkin research group for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jevtovic, V., Vidovic, D. Synthesis, Characterization and X-Ray Crystal Structure of the Tri Aqua (3-Hydroxy-5-Hydroxymethyl-2-Methylpyridine-4-Carboxaldehyde-3-Methylisotiosemicarbazone: k3, O3, N7, N10) Ni(II) Nitrate. J Chem Crystallogr 40, 794–798 (2010). https://doi.org/10.1007/s10870-010-9746-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-010-9746-2