Abstract
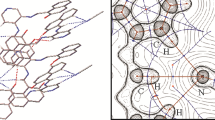
The crystal structure of the modified unsymmetrically N,N′-substituted viologen chromophore, N-ethyl-N′-(2-phosphonoethyl)-4,4′-bipyridinium dichloride 0.75 hydrate (1) has been determined. Crystals are triclinic, space group P−1 with Z = 2 in a cell with a = 7.2550(1), b = 13.2038(5), c = 18.5752(7) Å, α = 86.495(3), β = 83.527(2), γ = 88.921(2)°. The two independent but pseudo-symmetrically related cations in the asymmetric unit form one-dimensional hydrogen-bonded chains through short homomeric phosphonic acid O–H···O links [2.455(4), 2.464(4) Å] while two of the chloride anions are similarly strongly linked to phosphonic acid groups [O–H···Cl, 2.889(4), 2.896(4) Å]. The other two chloride anions together with the two water molecules of solvation (one with partial occupancy) form unusual cyclic hydrogen-bonded bis(Cl···water) dianion units which lie between the layers of bipyridylium rings of the cation chain structures with which they are weakly associated.
Graphical Abstract
The crystal structure determination of unsymmetrically substituted viologen N-ethyl-N′-(2-phosphonoethyl)-4,4′-bipyridylium dichloride 0.75 hydrate shows the presence of strong intermolecular phosphonate O–H···O and O–H···Cl interactions together with unusual cyclic hydrogen-bonded bis(chloride···water) dianion units giving one-dimensional chain structures.





Similar content being viewed by others
References
Michaelis L (1932) Biochem Z 250:564
Michaelis L, Hill ES (1933) J Am Chem Soc 55:1481
O’Neil MJ (ed) (2001) The Merck index, 13th edn. Merck & Co. Inc, Whitehouse Station, p 1782
Asakura N, Hiraishi T, Kamachi T, Okura I (2001) Mol Catal A Chem 172:1
Sakamoto M, Kamachi T, Okura I, Ueno A, Mihara H (2001) Biopolymers 59:103
Toba R, Maria Quintela J, Peinador C, Roman E, Kaifer AE (2001) Chem Commun 9:857
Suzuki M, Waraksa CC, Mallouk TE, Nakayama H, Hanabusa K (2002) J Phys Chem B 106:4227
Amao Y, Tomonou Y, Okura I (2003) Sol Energy Mater Sol Cells 79:103
Cinnsealach R, Boschloo G, Rao SN, Fitzmaurice D (1999) Sol Energy Mater Sol Cells 57:107
Sotomayor J, Will G, Fitzmaurice D (2000) J Mater Chem 10:685
Will G, Boschloo G, Nagaraja Rao S, Fitzmaurice D (1999) J Phys Chem B 103:8067
Will G, Nagaraja Rao JSS, Fitzmaurice D (1999) J Mater Chem 9:2297
Long B, Nikitin K, Fitzmaurice D (2003) J Am Chem Soc 125:15490
Russell JH, Wallwork SC (1972) Acta Crystallogr B28:1527
Cousson A, Bachet B, Kokel B, Hubert-Habart M (1993) Acta Crystallogr C49:942
Wolkers H, Stegmann R, Frenking G, Dehnicke K, Fenske D, Baum G (1993) Z Naturforsch B Chem Sci 48:1341
Argay G, Kalman A, Ribar B (1995) Z Kristallogr 210:455
Hu T (2009) Acta Crystallogr E65:o1162
Pettitt BM, Rossky PJ (1986) J Chem Phys 84:5836
Gao J, Boudon G, Wipff G (1991) J Am Chem Soc 113:9610
Bernstein J, Davis RE, Shimoni L, Chang N-L (1995) Angew Chem Int Ed Engl 34:1555
Kleinman EF, Bordner J, Newhouse BJ, MacFerrin K (1992) J Am Chem Soc 114:4945
Mak TCW (1984) Inorg Chem 23:620
Lentz BR, Scheraga HA (1969) J Chem Phys 50:5296
Wilson GJ (2006) PhD thesis, Queensland University of Technology, Brisbane, Australia
CrysAlis CCD, CrysAlis RED (2008) X-ray data acquisition and reduction programs. Oxford Diffraction Ltd, Abington (version 1.171.32.5)
Sheldrick GM (1996) SADABS, Absorption correction program for area detectors. University of Göttingen, Germany
Sheldrick GM (2008) Acta Crystallogr A64:112
Farrugia LJ (1999) J Appl Crystallogr 32:837
Spek AL (2009) Acta Crystallogr D65:148
Acknowledgments
The authors acknowledge financial support from the Australian Research Council, the School of Physical and Chemical Sciences (Queensland University of Technology) and the Commonwealth Scientific and Industrial Research Organisation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, G., Will, G.D. & Wilson, G.J. Hydrogen Bonding in the Water Stabilized Structure of the Modified Unsymmetrically Substituted Viologen Chromophore, N-ethyl-N′-(2-phosphonoethyl)-4,4′-bipyridylium Dichloride. J Chem Crystallogr 40, 248–252 (2010). https://doi.org/10.1007/s10870-009-9642-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-009-9642-9