Abstract
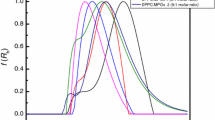
In this study, we address the effect of the cis-double bond in 1,2-dioleoyl-sn-glycero-3-phosphoethanolamide-N-[methoxy(polyethylene glycol)-2000, DOPE PEG2000 (DP), on the Langmuir monolayer of C18 fatty acids—namely, stearic acid (SA), oleic acid (L1), linoleic acid (L2), and linolenic acid (L3)—with the same head group but different degrees of saturation on their hydrocarbon chains. Negative values of Gibbs free energy of mixing (ΔG mix) were obtained throughout the investigated ranges of the unsaturated C18 fatty-acid (L1, L2 and L3) mixed systems, indicating that very strong attractions occurred between molecules in the monolayers. The bend and kink effects from the cis-double bond(s) in the hydrocarbon chain affected the membrane fluidity and molecular packing in the monolayers, which resulted in a greater interaction between unsaturated C18 fatty acids and DP. The most thermodynamically stable mole composition of unsaturated C18 fatty acids to DP was observed at 50:1; this ratio is suggested to be the best mole ratio and will be subsequently used to prepare DP–C18 fatty-acid nanoliposomes. The presence of cis-double bonds in both hydrocarbon chains of DOPE in DP also created an imperfection in the membrane structure of lipid-drug delivery systems, which is expected to enhance lipid-based systems for antibody conjugation and drug encapsulation.










Similar content being viewed by others
References
Chonn, A., Cullis, P.R.: Recent advances in liposome technologies and their applications for systemic gene delivery. Adv. Drug Deliv. Rev. 30, 73–83 (1998)
Allen, C., Santos, N.D., Gallagher, R., Chiu, G., Shu, Y., Li, W., Johnstone, S., Janoff, A., Mayer, L., Webb, M., Bally, M.: Controlling the physical behavior and biological performance of liposome formulations through use of surface grafted poly(ethylene) glycol. Biosci. Rep. 22, 225–250 (2002)
Moghimia, S.M., Szebenib, J.: Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog. Lipid Res. 42, 463–478 (2003)
Torchilin, V.P.: Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 4, 145–160 (2005)
Immordino, M.L., Dosio, F., Cattel, L.: Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomedicine. 1, 297–315 (2006)
Zhang, Y.: Stealth Liposomes: the silent nanobombers. Trends in Bio/Pharm. Industry. 19–24 (2008)
Chang, H.I., Yeh, M.K.: Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int. J. Nanomedicine 7, 49–60 (2012)
Nag, O.K., Awasthi, V.: Surface engineering of liposomes for stealth behavior. Pharmaceutics 5, 542–569 (2013)
Anselmo, A.C., Mitragotri, S.: An overview of clinical and commercial impact of drug delivery systems. J. Control. Release. 190, 15–28 (2014)
Ng, K.Y., Zhao, L., Liu, Y., Mahapatro, M.: The effects of polyethyleneglycol (PEG)-derived lipid on the activity of target-sensitive immunoliposome. Int. J. Pharm. 193, 157–166 (2000)
Yanga, T., Choi, M.K., Cuia, F.D., Kim, J.S., Chung, S.J., Shimb, C.K., Kim, D.D.: Preparation and evaluation of paclitaxel-loaded PEGylated immunoliposome. J. Control. Release 120, 169–177 (2007)
Manjappa, A.S., Chaudhari, K.R., Venkatarajua, M.P., Dantuluri, P., Nanda, B., Sidda, C., Sawant, K.K., Murthy, R.S.R.: Antibody derivatization and conjugation strategies: application in preparation of stealth immunoliposome to target chemotherapeutics to tumor. J. Control. Release 150, 2–22 (2011)
Gunaseelan, S., Gunaseelan, K., Zhang, M.D.X., Sinko, P.J.: Surface modifications of nanocarriers for effective intracellular delivery of anti-HIV drugs. Adv. Drug Deliv. Rev. 62, 518–531 (2010)
Working, P.K., Newman, M.S., Huang, S.K., Mayhew, E., Vaage, J., Lasic, D.D.: Pharmacokinetics, biodistribution and therapeutic efficacy of doxorubicin encapsulated in stealth® liposomes (Doxil®). J. Liposome Res. 4, 667–687 (1994)
Allen, T.M., Cullis, P.R.: Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 65, 36–48 (2013)
Blume, G., Cevc, G.: Molecular mechanism of the lipid vesicle longevity in vivo. Biochim. Biophys. Acta. 1146, 157–168 (1993)
Lee, A.G.: Lipid–protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta 1612, 1–40 (2003)
Seu, K.J., Cambrea, L.R., Everly, R.M., Hovis, J.S.: Influence of lipid chemistry on membrane fluidity: tail and headgroup interactions. Biophys. J. 91, 3727–3735 (2006)
Lingwood, L., Simons, K.: Lipid rafts as a membrane-organizing principle. Science. 327, 46–50 (2010)
Ma, G., Allen, H.C.: DPPC Langmuir monolayer at the air-water interface: probing the tail and head groups by vibrational sum frequency generation spectroscopy. Langmuir. 22, 5341–5349 (2006)
Lingwood, D., Simons, K.: Lipid rafts as a membrane-organizing principle. Science. 327, 46–50 (2010)
Barelli, H., Antonny, B.: Lipid unsaturation and organelle dynamics. Curr. Opin. Cell Biol. 41, 25–32 (2016)
Sezgin, E., Levental, I., Mayor, S., Eggeling, C.: The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 18(6), 361–374 (2017). doi:10.1038/nrm.2017.16
Johnston, M.J., Semple, S.C., Klimuk, S.K., Ansell, S., Maurer, N., Cullis, P.R.: Characterization of the drug retention and pharmacokinetic properties of liposomal nanoparticles containing dihydrosphingomyelin. Biochim. Biophys. Acta 1768, 1121–1127 (2007)
Bestman-Smith, J., Gourde, P., Desormeaux, A., Tremblay, M.J., Bergeron, M.G.: Sterically stabilized liposomes bearing anti-HLA-DR antibodies for targeting the primary cellular reservoirs of HIV-1. Biochim. Biophys. Acta. 1468, 161–174 (2000)
Loomis, K., Smith, B., Feng, Y., Garg, H., Yavlovich, A., Campbell-Massa, R., Dimitrov, D.S., Blumenthal, R., Xiao, X., Puri, A.: Specific targeting to B cells by lipid-based nanoparticles conjugated with a novel CD22-ScFv. Exp Mol. Pathol. 88, 238–249 (2010)
Lehtinen, J., Raki, M., Bergström, K.A., Uutela, P., Lehtinen, K., Hiltunen, A., Pikkarainen, J., Liang, H., Pitkänen, S., Määttä, A.M., Ketola, R.A., Yliperttula, M., Wirth, T., Urtti, A.: Pre-targeting and direct immunotargeting of liposomal drug carriers to ovarian carcinoma. PLoS ONE 7, 1–10 (2012)
Yan, F., Li, L., Deng, Z., Jin, Q., Chen, J., Yang, W., Yeh, C.K., Wu, J., Shandas, R., Liu, X., Zheng, H.: Paclitaxel-liposome-microbubble complexes as ultrasound-triggered therapeutic drug delivery carriers. J. Control. Release 166, 246–255 (2013)
Kroon, J., Metselaar, J.M., Storm, G., van der Pluijm, G.: Liposomal nanomedicines in the treatment of prostate cancer. Cancer Treat. Rev. 40, 578–584 (2014)
Foreman, M.B., Coffman, J.P., Murcia, M.J., Cesana, S., Jordan, R., Smith, G.S., Naumann, C.A.: Gelation of amphiphilic lipopolymers at the air−water interface: 2D analogue to 3D gelation of colloidal systems with grafted polymer chains. Langmuir 19, 326–332 (2003)
Cavalcanti, L.P., Tho, I., Konovalov, O., Fossheim, S., Brandl, M.: Compressibility study of quaternary phospholipid blend monolayers. Colloids Surf. B 85, 153–160 (2011)
Yoshizawa, Y., Kono, Y., Ogawara, K.I., Kimura, T., Higaki, K.: PEG liposomalization of paclitaxel improved its in vivo disposition and anti-tumor efficacy. Int. J. Pharm. 412, 132–141 (2011)
Gaillard, P.J., Appeldoorn, C.C.M., Dorland, R., van Kregten, J., Manca, F., Vugts, D.J., Windhorst, B., van Dongen, G.A., de Vries, H.E., Maussang, D., van Tellingen, O.: Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin. PLoS ONE 9, 1–10 (2014)
Lundberg, B.B., Griffiths, G., Hansen, H.J.: Cellular association and cytotoxicity of anti-74-targeted lipid drug carriers in B lymphoma cells. J. Control. Release 94, 155–161 (2004)
Lu, R.M., Chen, M.S., Chang, D.K., Chiu, C.Y., Lin, W.C., Yan, S.L., Wang, Y.P., Kuo, Y.S., Yeh, C.Y., Lo, A., Wu, H.C.: Targeted drug delivery systems mediated by a novel peptide in breast cancer therapy and imaging. PLoS ONE 8, 1–10 (2013)
Girard-Egrot, A.P., Godoy, S., Blum, L.J.: Enzyme association with lipidic Langmuir–Blodgett films: interests and applications in nanobioscience. Adv. Colloid Interf. Sci. 116, 205–225 (2005)
Wydro, P., Krajewska, B., Hąc-Wydro, K.: Chitosan as a lipid binder: a Langmuir monolayer study of chitosan–lipid interactions. Biomacromolecules 8, 2611–2617 (2007)
Hąc-Wydro, K., Jędrzejek, K., Dynarowicz-Łątka, P.: Effect of saturation degree on the interactions between fatty acids and phosphatidylcholines in binary and ternary Langmuir monolayers. Colloids Surf. B 72, 101–111 (2009)
Hąc-Wydro, K., Wydro, P.: The influence of fatty acids on model cholesterol/phospholipid membranes. Chem. Phys. Lipids 150, 66–81 (2007)
Gew, L.T., Misran, M.: Albumin-fatty acid interactions at monolayer interface. Nanoscale Res. Lett. 9, 218 (2014). doi:10.1186/1556-276X-9-218
Crawford, N.F., Leblanc, R.M.: Serum albumin in 2D: a Langmuir monolayer approach. Adv. Colloid Interf. Sci. 207, 131–138 (2014)
Needham, D., Kim, D.H.: PEG-covered lipid surfaces: bilayers and monolayers. Colloids Surf. B 18, 183–195 (2000)
Sriwongsitanont, S., Ueno, M.: Physicochemical properties of PEG-grafted liposomes. Chem. Pharm. Bull. 50(9), 1238–1244 (2002)
Rovira-Bru, M., Thompson, D.H., Szleifer, I.: Size and structure of spontaneously forming liposomes in lipid/PEG-lipid mixtures. Biophys. J. 83(5), 2419–2439 (2002)
Garbuzenko, O., Barenholz, Y., Priev, A.: Effect of grafted PEG on liposome size and on compressibility and packing of lipid bilayer. Chem. Phys. Lipids 150, 66–81 (2005)
Gew, L.T., Misran, M.: Energetic mixing of anti-SNAP25 on lipid monolayers: degree of saturation of C18 fatty acids. Surf. Interface Anal. 49(5), 388–397 (2016). doi:10.1002/sia.6144
Davies, J.T., Rideal, E.K.: Interfacial Phenomena. Academic Press, New York (1963)
Gaines, G.L.: Insoluble Monolayers at Liquid-Gas Interfaces. Interscience, New York (1966)
Gupta, R.K., Manjuladevi, V.: Molecular interactions at interfaces. In: Aurelia, M. (ed.) Molecular Interactions, pp 81–104. InTech Open Access Publisher, Crotia (2012)
Buys, A.V., Rooy, M.J.V., Soma, P., Papendorp, D.V., Lipinski, B., Pretorius, E.: Change in red blood cell membrane structure in type 2 diabetes: a scanning electron and atomic force microscopy study. Cardiovasc. Diabetol. 12, 25 (2013)
Abednejab, A.S., Amoabediny, G., Ghaee, A.: Surface modification of polypropylene membrane by polyethylene glycol graft polymerization. Mater. Sci. Eng. C 42, 443–450 (2014)
Johnson, D., Hilal, N.: Characterisation and quantification of membrane surface properties using atomic force microscopy: a comprehensive review. Desalination 356, 149–164 (2015)
De Oliveira, R.R.L., Albuquerque, D.A.C., Cruz, T.G.S., Yamaji, F.M., Leite, F.L.: Measurement of the nanoscale roughness by atomic force microscopy: basic principles and applications. In: Victor, B. (ed.) Atomic Force Microscopy - Imaging, Measuring and Manipulating Surfaces at the Atomic Scale, pp. 147–175. INTECH Open Access Publisher, Croatia (2012)
Acknowledgements
This study was financially supported by the Fundamental Research Grant Scheme (FP013-2015A) and UMRG Flagship (RP022C-16SUS), Malaysia. Gew would like to express her thanks to Phra Phrom for his blessing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Gew, L.T., Misran, M. Interaction between C18 fatty acids and DOPE PEG2000 in Langmuir monolayers: effect of degree of unsaturation. J Biol Phys 43, 397–414 (2017). https://doi.org/10.1007/s10867-017-9459-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-017-9459-2