Abstract
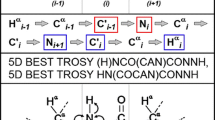
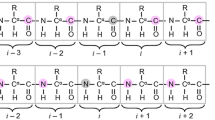
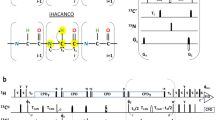
NMR spectroscopy is by far the most versatile and information rich technique to study intrinsically disordered proteins (IDPs). While NMR is able to offer residue level information on structure and dynamics, assignment of chemical shift resonances in IDPs is not a straightforward process. Consequently, numerous pulse sequences and assignment protocols have been developed during past several years, targeted especially for the assignment of IDPs, including experiments that employ HN, Hα or 13C detection combined with two to six indirectly detected dimensions. Here we propose two new HN-detection based pulse sequences, (HCA)CON(CAN)H and (HCA)N(CA)CO(N)H, that provide correlations with 1HN(i − 1), 13C′(i − 1) and 15N(i), and 1HN(i + 1), 13C′(i) and 15N(i) frequencies, respectively. Most importantly, they offer sequential links across the proline bridges and enable filling the single proline gaps during the assignment. We show that the novel experiments can efficiently complement the information available from existing HNCO and intraresidual i(HCA)CO(CA)NH pulse sequences and their concomitant usage enabled >95 % assignment of backbone resonances in cytoplasmic tail of adenosine receptor A2A in comparison to 73 % complete assignment using the HNCO/i(HCA)CO(CA)NH data alone.




Similar content being viewed by others
References
Bai Y, Milne JS, Mayne L, Englander SW (1993) Primary structure effects on peptide group hydrogen exchange. Proteins Struct Funct Genet 17:75–86
Bermel W, Bertini I, Felli IC, Piccioli M, Pierattelli R (2006) 13C-detected protonless NMR spectroscopy of proteins in solution. Prog Nucl Magn Reson Spectr 48:25–45
Bermel W, Bertini I, Csizmok V, Felli IC, Pierattelli R, Tompa P (2009) H-start for exclusively heteronuclear NMR spectroscopy: the case of intrinsically disordered protein. J Magn Reson 198:275–281
Bermel W, Bertini I, Felli IC, Gonnelli L, Kozminski W, Piai A, Pierattelli R, Stanek J (2012) Speeding up sequence specific assignment of IDPs. J Biomol NMR 53:293–301
Bottomley MJ, Macias MJ, Liu Z, Sattler M (1999) A novel NMR experiment for the sequential assignment of proline residues and proline stretches in 13C/15N-labeled proteins. J Biomol NMR 13:381–385
Brutscher B (2002) Intraresidue HNCA and COHNCA experiments for protein backbone resonance assignment. J Magn Reson 156:155–159
Delaglio F, Torchia DA, Bax A (1991) Measurement of 15N-13C J couplings in staphylococcal nuclease. J Biomol NMR 1:439–446
Dyson HJ, Wright PE (2001) Nuclear magnetic resonance methods for elucidation of structure and dynamics in disordered states. Methods Enzymol 339:258–270
Dyson HJ, Wright PE (2005) Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol 6:197–208
Fiorito F, Hiller S, Wider G, Wüthrich K (2006) Automated resonance assignment of proteins: 6D APSY-NMR. J Biomol NMR 35:27–37
Grzesiek S, Bax A (1993) Amino acid type determination in the sequential assignment procedure of uniformly 13C/15N-enriched proteins. J Biomol NMR 3:185–204
Grzesiek S, Bax A, Hu J-S, Kaufman J, Palmer I, Stahl SJ, Tjandra N, Wingfield PT (1997) Refined solution structure and backbone dynamics of HIV-1 Nef. Protein Sci 6:1248–1263
Hellman M, Tossavainen H, Rappu P, Heino J, Permi P (2011) Characterization of intrinsically disordered prostate associated gene (PAGE5) at single residue resolution by NMR spectroscopy. PLoS One 6:e26633
Hu K, Vögeli B, Clore GM (2007) Spin-state selective carbon-detected HNCO with TROSY optimization in all dimensions and double echo-antiecho sensitivity enhancement in both indirect dimensions. J Am Chem Soc 129:5484–5491
Kanelis V, Donaldson L, Muhandiram DR, Rotin D, Forman-Kay JD, Kay LE (2000) Sequential assignment of proline-rich regions in proteins: application to modular binding domain complexes. J Biomol NMR 16:253–259
Kay LE, Keifer P, Saarinen T (1992) Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J Am Chem Soc 114:10663–10665
Kazimierczuk K, Stanek J, Zawadzka-Kazimierczuk A, Kozminski W (2013) High-dimensional NMR spectra for structural studies of biomolecules. ChemPhysChem 14:3015–3025
Kosol S, Contreras-Martos S, Cedeno C, Tompa P (2013) Structural characterization of intrinsically disordered proteins by NMR spectroscopy. Molecules 18:10802–10828
Kumar D, Hosur RV (2011) hNCOcanH pulse sequence and a robust protocol for rapid and unambiguous assignment of backbone (1HN, 15N and 13C′) resonances in 15N/13C-labeled proteins. Magn Reson Chem 49:575–583
Kupće E, Wagner G (1995) Wideband homonuclear decoupling in protein spectra. J Magn Reson 109A:329–333
Logan TM, Olejniczak ET, Xu RX, Fesik SW (1993) A general method for assigning NMR spectra of denatured proteins using 3D HC(CO)NH-TOCSY triple resonance experiments. J Biomol NMR 3:225–231
Löhr F, Pfeiffer S, Lin Y-J, Hartleib J, Klimmek O, Rüterjans H (2000) HNCAN pulse sequences for sequential backbone resonance assignment across proline residues in perdeuterated proteins. J Biomol NMR 18:337–346
Mäntylahti S, Hellman M, Permi P (2011) Extension of the HA-detection based approach: (HCA)CON(CA)H and (HCA)NCO(CA)H experiments for the main-chain assignment of intrinsically disordered proteins. J Biomol NMR 49:99–109
Mäntylahti S, Tossavainen H, Hellman M, Permi P (2009) An intraresidual i(HCA)CO(CA)NH experiment for the assignment of main-chain resonances in 15N, 13C labeled proteins. J Biomol NMR 45:301–310
Mäntylahti S, Aitio O, Hellman M, Permi P (2010) HA-detected experiments for the backbone assignment of intrinsically disordered proteins. J Biomol NMR 47:171–181
Marion D, Ikura M, Tschudin R, Bax A (1989) Rapid recording of 2D NMR-spectra without phase cycling—application to the study of hydrogen-exchange in proteins. J Magn Reson 85:393–399
Morris GA, Freeman R (1979) Enhancement of nuclear magnetic resonance signals by polarization transfer. J Am Chem Soc 101:760–762
Muhandiram DR, Kay LE (1994) Gradient-enhanced triple-resonance three-dimensional NMR experiments with improved sensitivity. J Magn Reson 103:203–216
Nietlispach D (2002) A novel approach for the sequential backbone assignment of larger proteins: selective intra-HNCA and DQ-HNCA. J Am Chem Soc 124:11199–11207
Nováček J, Zawadzka-Kazimierczuk A, Papoušková V, Zídek L, Sanderová H, Krásný L, Koźmiński W, Sklenář V (2011) 5D 13C-detected experiments for backbone assignment of unstructured proteins with a very low signal dispersion. J Biomol NMR 50:1–11
Nováček J, Haba NY, Chill JH, Zídek L, Sklenář V (2012) 4D non-uniformly sampled HCBCACON and 1J(NCα)-selective HCBCANCO experiments for the sequential assignment and chemical shift analysis of intrinsically disordered proteins. J Biomol NMR 53:139–148
Panchal SC, Bhavesh NS, Hosur RV (2001) Improved 3D triple resonance experiments, HNN and HN(C)N, for H-N and N-15 sequential correlations in (C-13, N-15) labeled proteins: application to unfolded proteins. J Biomol NMR 20:135–147
Pantoja-Uceda D, Santoro J (2013) Direct correlation of consecutive C′-N groups in proteins: a method for the assignment of intrinsically disordered proteins. J Biomol NMR 57:57–63
Permi P (2002) Intraresidual HNCA: an experiment for correlating only intraresidual backbone resonances. J Biomol NMR 23:201–209
Permi P, Annila A (2004) Coherence transfer in proteins. Prog Nucl Magn Reson Spectr 44:97–137
Permi P, Hellman M (2012) Alpha proton detection based backbone assignment of intrinsically disordered proteins. Methods Mol Biol 895:211–226
Rios CB, Feng W, Tashiro M, Shang Z, Montelione GT (1996) Phase labeling of C–H and C–C spin-system topologies: application in constant-time PFG-CBCA(CO)NH experiments for discriminating amino acid spin-system types. J Biomol NMR 8:345–350
Sattler M, Schleucher J, Griesinger C (1999) Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog Nucl Magn Reson Spectr 34:93–158
Schleucher J, Schwendinger MG, Sattler M, Schmidt P, Glaser SJ, Sørensen OW, Griesinger C (1994) A general enhancement scheme in heteronuclear multidimensional NMR employing pulsed-field gradients. J Biomol NMR 4:301–306
Shaka AJ (1985) Composite pulses for ultra-broadband spin inversion. Chem Phys Lett 120:201–205
Shaka AJ, Keeler J, Frenkiel T, Freeman R (1983) An improved sequence for broad-band decoupling—Waltz-16. J Magn Reson 52:335–338
Tossavainen H, Permi P (2004) Optimized pathway selection in intraresidual triple-resonance experiments. J Magn Reson 170:244–251
Uversky VN (2013) A decade and a half of protein intrinsic disorder: biology still waits for physics. Protein Sci 22:693–724
Yao J, Dyson HJ, Wright PE (1997) Chemical shift dispersion and secondary structure prediction in unfolded and partly folded proteins. FEBS Lett 419:285–289
Acknowledgments
This work was financially supported by the Grants 259447 (to P. P.) and 132138 (to V. P. J.) from the Academy of Finland, and by the IRG 249081 from FP7 Marie Curie European Reintegration Grant and Biocenter Oulu (to V. P. J.). Biocenter Finland and Biocentrum Helsinki are acknowledged for the infrastructure support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hellman, M., Piirainen, H., Jaakola, VP. et al. Bridge over troubled proline: assignment of intrinsically disordered proteins using (HCA)CON(CAN)H and (HCA)N(CA)CO(N)H experiments concomitantly with HNCO and i(HCA)CO(CA)NH. J Biomol NMR 58, 49–60 (2014). https://doi.org/10.1007/s10858-013-9804-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-013-9804-0