Abstract
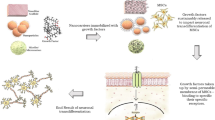
Sustained and controlled release of neurotrophic factors in target tissue through nanomaterial based delivery system could be a better strategy for nerve tissue regeneration. The present study aims to prepare the nerve growth factor (NGF) encapsulated chitosan nanoparticles (NGF-CNPs) and its evaluation on neuronal differentiation potentiality of canine bone marrow derived mesenchymal stem cells (cBM-MSCs). The NGF-CNPs were prepared by ionotropic gelation method with tripolyphosphate (TPP) as an ionic cross-linking agent. Observations on physiochemical properties displayed the size of nanoparticles as 80–90 nm with positive zeta potential as well as an ionic interaction between NGF and nanoparticle. NGF loading efficiency was found to be 61% while its sustained release was observed by an in vitro release kinetics study. These nanoparticles were found to be cytocompatible to cBM-MSCs when supplemented at a concentration upto 4 mg/ml in culture media. The NGF-CNP supplemented culture media was able to transdifferentiate the preinduced cBM-MSCs into neurons in a better way than unbound NGF supplementation. Further, it was also noticed that NGF-CNPs were able to transdifferentiate cBM-MSCs without any chemical based preinduction. In conclusion, our findings propose that NGF-CNPs are capable of releasing bioactive NGF with the ability to transdifferentiate mesenchymal stem cells into neurons, suggesting its potential future application in nerve tissue regeneration.
Graphical abstract






Similar content being viewed by others
References
Tropel P, Platet N, Platel JC, Noël D, Albrieux M, Benabid AL, Berqer F. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:2868–76.
Guilak F, Lott KE, Awad HA, Cao Q, Hicok KC, Fermor B, Gimble JM. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Phys. 2006;206:229–37.
Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353–64.
Jung D, Ha J, Kang BT, Kim JW, Quan FS, Lee JH, Woo EJ, Park HM. A comparison of autologous and allogenic bone marrow derived mesenchymal stem cell transplantation in canine spinal cord injury. J Neurol Sci. 2009;285:67–77.
Yang Q, Mu J, Li Q, Li A, Zeng Z, Yang J, Zhang X, Tang J, Xie P. A simple and efficient method for deriving neurospheres from bone marrow stromal cells. Biochem Biophys Res Commun. 2008;372:520–4.
Kim KN, Oh SH, Lee KH, Yoon DH. Effect of human mesenchymal stem cell transplantation combined with growth factor infusion in the repair of injured spinal cord. Acta Neurochir Suppl. 2006;99:133–6.
Lim JH, Byeon YE, Ryu HH, Jeong YH, Lee YW, Kim WH, Kang KS, Kweon OK. Transplantation of canine umbilical cord blood derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci. 2007;8:275–82.
Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD, et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–11.
Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–49.
Boyd JG, Gordon T. Glial cell line-derived neurotrophic factor and brainderived neurotrophic factor sustain the axonal regeneration of chronically axotomized motoneurons in vivo. Exp Neurol. 2003;183:610–9.
Zhao LX, Zhang J, Cao F, Meng L, Wang DM, Li YH, Jiao WC, Zheng M, Xu XH, Pei XT. Modification of the brain-derived neurotrophic factor gene: a portal to transform mesenchymal stem cells into advantageous engineering cells for neuroregeneration and neuroprotection. Exp Neurol. 2004;190:396–406.
Naghdi M, Tiraihi T, Namin SAM, Arabkheradmand J. Transdifferentiation of bone marrow stromal cells into cholinergic neuronal phenotype: a potential source for cell therapy in spinal cord injury. Cytotherapy. 2009a;11:137–52.
Naghdi M, Tiraihi T, Namin SAM, Arabkheradmand J. Induction of bone marrow stromal cells into cholinergic-like cells by nerve growth factor. Iran Biomed J. 2009b;2:117–23.
Lim JY, Park SI, Kim SM, Jun JA, Oh JH, Ryu CH, Jeong CH, Park SH, Park SA, Oh W, Chang JW, Jeun SS. Neural differentiation of brain-derived neurotrophic factor-expressing human umbilical cord blood-derived mesenchymal stem cells in culture via TrkB-mediated ERK and β-catenin phosphorylation and following transplantation into the developing brain. Cell Transpl. 2011;20:1855–66.
Tsai C, Lu MC, Chen YS, Wu CH, Lin C. Locally administered nerve growth factor suppresses ginsenoside Rb1-enhanced peripheral nerve regeneration. Am J Chin Med. 2003;31:665–73.
Zeng W, Huang J, Hu X, Xiao W, Rong M, Yuan Z, Luo Z. Ionically cross-linked chitosan microspheres for controlled release of bioactive nerve growth factor. Int J Pharm. 2011;421:283–90.
Dyondi D, Webster TJ, Banerjee R. A nanoparticulate injectable hydrogel as a tissue engineering scaffold for multiple growth factor delivery for bone regeneration. Int J Nanomed. 2013;8:47–59.
Li X, Yang Z, Zhang A, Wang T, Chen W. Repair of thoracic spinal cord injury by chitosan tube implantation in adult rats. Biomaterials. 2009;30:1121–32.
Lu GY, Kong LJ, Sheng BY, Wang G, Gong YD, Zhang XF. Degradation of covalently cross-linked carboxymethyl chitosan and its potential application for peripheral nerve regeneration. Eur Polym J. 2007;43:3807–18.
Huang J, Hu X, Lu L, Ye Z, Zhang Q, Luo Z. Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers. J Biomed Mater Res A. 2010a;93:164–74.
Huang J, Hu X, Lu L, Ye Z, Zhang Q, Luo Z. Electrical stimulation accelerates motor functional recovery in the rat model of 15 mm sciatic nerve gap bridged by scaffolds with longitudinally oriented micro-channels. Neural Repair. 2010b;24:736–45.
Lee AC, Yu VM, Lowe JB, Brenner MJ, Hunter DA, Mackinnon SE, Sakiyama-Elbert SE. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol. 2003;184:295–303.
Yu H, Liu J, Ma J, Xiang L. Local delivery of controlled released nerve growth factor promotes sciatic nerve regeneration after crush injury. Neurosci Lett. 2014;566:177–81.
Bhang SH, Lee TJ, Lim JM, Lim JS, Han AM, Choi CY, Kwon YH, Kim BS. The effect of the controlled release of nerve growth factor from collagen gel on the coeffiiency of neural cell culture. Biomaterials. 2009;30:126–32.
Cho Y, Choi JS, Jeong SY, Yoo HS. Nerve growth factor (NGF)-conjugated electrospun nanostructures with topgraphical cues for neuronal differentiation of mesenchymal stem cells. Acta Biomater. 2010;6:4725–33.
LuZhong Z, YouLang Z, GuiCai L, YaHong Z, XiaoSong G, YuMin Y. Nanoparticle mediated controlled delivery of dual growth factors. Sci China Life Sci. 2014;57:256–62.
Carrello Gomez B, Duncan R. Evaluation of the biological properties of soluble chitosan and chitosan microsphere. Int J Pharm. 1997;148:231–40.
Richardson SCW, Kolbe HVJ, Duncan R. Potential of low molecular mass chitosan as a DNA delivery system: biocompatibility, body distribution and ability to complex and protect DNA. Int J Pharm. 1999;178:231–43.
Katas H, Alpar HO. Development and characterisation of chitosan nanoparticles for siRNA delivery. J Cont Release. 2006;115:216–25.
Csaba N, Koping Hoggard M, Alonso MJ. Ionically cross linked chitosan/tripolyphosphate nanoparticles for oligonucleotide and plasmid DNA delivery. Int J Pharm. 2009;382:205–14.
DeJong W, Hagens W, Krystek P, Burger M, Sips A, Geertsma RE. Particle size dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–9.
Liu TG, Li B, Huang W, Lv B, Chen J, Zhang JX, Zhu LP. Effects and kinetics of a novel temperature cycling treatment on the N-deacetylation of chitin in alkaline solution. Carbohydr Polym. 2009;77:110–7.
Jayakumar R, Chennazhi KP, Muzzarelli RAA, Tamura H, Nair SV, Selvamurugan N. Chitosan conjugated DNA nanoparticles in gene therapy. Carbohydr Polym. 2010;79:1–8.
Khemkhao M, Nuntakumjorn B, Techkarnjanaruk S, Phalakornkule C. Effect of chitosan on UASB treating POME during a transition from mesophilic to thermophilic conditions. Bioresour Technol. 2011;102:4674–81.
Park JS, Yang NHN, Woo DG, Jeon SY, Park KH. The promotion of chondrogenesis, osteogenesis, and adipogenesis of human mesenchymal stem cells by multiple growth factors incorporated into nanosphere-coated microspheres. Biomaterials. 2011;32:28–38.
Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent, applications and mode of action. Biomacromolecules. 2003;4:1457–65.
Hirano S. Chitin and chitosan as novel biotechnological materials. Polym Int. 1999;48:732–4.
Kurita K. Chitin and chitosan: functional biopolymers from marine crustaceans. Mar Biotechnol. 2006;8:203–26.
Yi H, Wu LQ, Bentley WE, Ghodssi R, Rubloff GW, Culver JN, Payne GF. Biofabrication with chitosan. Biomacromolecules. 2005;6:2881–94.
Mourya VK, Inamdar NN. Chitosan-modifications and applications: opportunities galore. React Funct Polym. 2008;68:1013–51.
Yang Y, Liu M, Gu Y, Lin S, Ding F, Gu X. Effect of chitooligosaccharide on neuronal differentiation of PC-12 cells. Cell Biol Int. 2009;33:352–6.
Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–70.
Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–56.
Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001;282:148–52.
Pan Y, Li YJ, Zhao HY, Zheng JM, Xu H, Wei G, Hao JS, Cui FD. Bioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int J Pharm. 2002;249:139–47.
Cetin M, Aktas Y, Vural I, Capan Y, Dogan LA, Duman M, Dalkara T. Preparation and in vitro evaluation of bFGF-loaded chitosan nanoparticles. Drug Deliv. 2007;14:525–9.
Pfaffl MW. A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acid Res. 2001;29:e45.
Houweling DA, Van Asseldonk JT, Lankhorst AJ, Hamers FP, Martin D, Bär PR, Joosten EA. Local application of collagen containing brain-derived neurotrophic factor decreases the loss of function after spinal cord injury in the adult rat. Neurosci Lett. 1998;251:193–6.
Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci. 2002;99:2199–204.
Pulavendran S, Rose C, Mandal AB. Hepatocyte growth factor incorporated chitosan nanoparticles differentiate murine bone marrow mesenchymal stem cells into hepatocytes in vitro. IET Nanobiotechnol. 2010;4:51–60.
Rampino A, Borgogna M, Blasi P, Bellich B, Cesaro A. Chitosan nanoparticles: preparation, size evaluation and stability. Int J Pharm. 2013;455:219–33.
Siterberg J, Özcetin A, Ehrhardt C, Bakowsky U. Utilising atomic force microscopy for the characterisation of nanoscale drug delivery systems. Eur J Pharm Biopharm. 2010;74:2–13.
Couvreur P, Barratt G, Fattal E, Legrand P, Vauthier C. Nanocapsule technology: a review. Crit Rev Ther Drug Carr Syst. 2002;19:99–134.
Janes KA, Alonso MJ. Depolymerized chitosan nanoparticles for protein delivery: preparation and characterization. J Appl Polym Sci. 2003;88:2769–76.
Fan W, Yan W, Xu Z, Ni H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf B Biointerfaces. 2012;90:21–27.
Rajam M, Pulavendran S, Rose C, Mandal AB. Chitosan nanoparticles as a dual growth factor delivery system for tissue engineering applications. Int J Pharm. 2011;410:145–52.
Stoica R, Şomoghia R, Iona RM. Preparation of chitosan–tripolyphosphate nanoparticles for the encapsulation of polyphenols extracted from rose hips. Dig J Nanomater Biostructures. 2013;8:955–63.
Schipper NG, Varum KM, Artursson P. Chitosans as absorption enhancers for poorly absorbable drugs. 1: influence of molecular weight and degree of acetylation on drug transport across human intestinal epithelial (CaCo-2) cells. Pharm Res. 1996;13:1686–92.
Schipper NG, Varum KM, Stenberg P, Ocklind G, Lennernas H, Artursson P. Chitosans as absorption enhancers of poorly absorbable drugs. 3: Influence of mucus on absorption enhancement. Eur J Pharm Sci. 1999;8:335–43.
Rao SB, Sharma CP. Use of chitosan as a biomaterial: studies on its safety and hemostatic potential. J Biomed Mater Res. 1997;34:21–28.
Illum L, Farraj NF, Davis SS. Chitosan as a novel nasal delivery system for peptide drugs. Pharm Res. 1994;11:1186–9.
Chatelet C, Damour O, Domard A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials. 2001;22:261–8.
Yang L, Watts DJ. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol Lett. 2005;158:122–32.
Donaldson K, Brown D, Clouter A, Duffin R, MacNee W, Renwick L, Tran L, Stone V. The pulmonary toxicology of ultrafine particles. J Aerosol Med. 2002;15:213–20.
Mostafalou S, Mohammadi H, Ramazani A, Abdollahi M. Different biokinetics of nanomedicines linking totheir toxicity: an overview. Daru. 2013;21:14.
Park JH, Cho YW, Chung H, Kwon IC, Jeong SY. Synthesis and characterization of sugar-bearing chitosan derivatives: aqueous solubility and biodegradability. Biomacromolecules. 2003;4:1087–91.
Teo KK, Yim EKF. Nano-patterned poly-ε-caprolactone with controlled release of retinoic acid and nerve growth factor for neuronal regeneration. IFMBE Proc. 2009;23:1348–51.
Zhang N, Wimmer J, Qian SJ, Chen WS. Stem cells: current approach and future prospects in spinal cord injury repair. Anat Rec. 2010;293:519–30.
Neuhuber B, Gallo G, Howard L, Kostura L, Mackay A, Fisher I. Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: disruption of actin cytoskeleton induces rapid morphological changes and mimics neuronal phenotype. J Neurosci Res. 2004;77:192–204.
Lu P, Blesch A, Tuszynski MH. Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact? J Neurosci Res. 2004;77:174–91.
Nakano R, Edamura K, Nakayama T, Teshima K, Asano K, Narita T, Okabayashi K, Sugiya H. Differentiation of canine bone marrow stromal cells into voltage- and glutamate-responsive neuron-like cells by basic fibroblast growth factor. J Vet Med Sci. 2014;77:27–35.
Redhead HM, Davis SS, Illum L. Drug delivery in poly (lactide-co-glycolide) nanoparticles surface modified with poloxamer 407 and poloxamine 908: in vitro characterisation and in vivo evaluation. J Control Release. 2001;70:353–63.
Corradetti B, Freile P, Pells S, Bagnaninchi P, Park J, Fahmy TM, de Sousa PA. Paracrine signalling events in embryonic stem cell renewal mediated by affinity targeted nanoparticles. Biomaterials. 2012;33:6634–43.
Del Puerto HL, Martins AS, Moro L, Milsted A, Alves F, Braz GF, Vasconcelos AC. Caspase3-/-8/-9 Bax and Bcl-2 expression in the cerebellum, lymph nodes and leukocytes ofdogs naturally infected with canine distemper virus. Genet Mol Res. 2010;269:151–61.
Wilcox JT, Lai JK, Semple E, Brisson BA, Gartley C, Armstrong JN, Betts DH. Synaptically-competent neurons derived from canine embryonic stem cells by lineage selection with EGF and noggin. PLoS One. 2011;6:e19768.
Vieira NM, Brandalise V, Zucconi E, Secco M, Strauss BE, Zatz M. Isolation, characterization and differentiation potential of canine adipose-derived stem cell. Cell Transpl. 2010;19:279–89.
Acknowledgements
The authors are thankful to Director, ICAR-Indian Veterinary Research Institute, Izatnagar-243 122, Bareilly, U.P., India for providing the facilities in carrying out this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Mili, B., Das, K., Kumar, A. et al. Preparation of NGF encapsulated chitosan nanoparticles and its evaluation on neuronal differentiation potentiality of canine mesenchymal stem cells. J Mater Sci: Mater Med 29, 4 (2018). https://doi.org/10.1007/s10856-017-6008-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-6008-2