Abstract
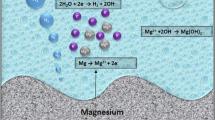
Magnesium (Mg) alloys are being actively investigated as potential load-bearing orthopaedic implant materials due to their biodegradability in vivo. With Mg biomaterials at an early stage in their development, the screening of alloy compositions for their biodegradation rate, and hence biocompatibility, is reliant on cost-effective in vitro methods. The use of a buffer to control pH during in vitro biodegradation is recognised as critically important as this seeks to mimic pH control as it occurs naturally in vivo. The two different types of in vitro buffer system available are based on either (i) zwitterionic organic compounds or (ii) carbonate buffers within a partial-CO2 atmosphere. This study investigated the influence of the buffering system itself on the in vitro corrosion of Mg. It was found that the less realistic zwitterion-based buffer did not form the same corrosion layers as the carbonate buffer, and was potentially affecting the behaviour of the hydrated oxide layer that forms on Mg in all aqueous environments. Consequently it was recommended that Mg in vitro experiments use the more biorealistic carbonate buffering system when possible.





Similar content being viewed by others
References
Witte F. The history of biodegradable magnesium implants: a review. Acta Biomater. 2010;6(5):1680–92.
Staiger MP, Pietak AM, Huadmai J, Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27(9):1728–34.
ISO 10993. Biological evaluation of medical devices—Part 5: tests for in vitro cytotoxicity. 2009.
ISO 10993. Biological evaluation of medical devices—Part 12: sample preparation and reference materials. 2007.
Fischer J, Pröfrock D, Hort N, Willumeit R, Feyerabend F. Improved cytotoxicity testing of magnesium materials. Mater Sci Eng B. 2011;176(11):830–4. doi:10.1016/j.mseb.2011.04.008.
Ng WF, Chiu KY, Cheng FT. Effect of pH on the in vitro corrosion rate of magnesium degradable implant material. Mater Sci Eng C. 2010;30(6):898–903.
Gray-Munro JE, Seguin C, Strong M. Influence of surface modification on the in vitro corrosion rate of magnesium alloy AZ31. J Biomed Mater Res A. 2009;91A(1):221–30. doi:10.1002/jbm.a.32205.
Apkon M. Cellular physiology of skeletal, cardiac and smooth muscle. In: Boron WF, Boulpaep EL, editors. Medical physiology. 2nd ed. New York: Saunders; 2008.
Hall JE. Guyton and hall textbook of medical physiology. 11th ed. Amsterdam: Elsevier; 2010.
Helgason CD, Miller CL. Basic cell culture protocols. 3rd ed. Methods in molecular biology. Totowa, NJ: Humana Press; 2005.
Good NE, Winget GD, Winter W, Connolly TN, Izawa S, Singh MM. Hydrogen ion buffers for biological research. Biochemistry. 1966;5(2):467–77.
Yamamoto A, Hiromoto S. Effect of inorganic salts, amino acids and proteins on the degradation of pure magnesium in vitro. Mater Sci Eng C. 2009;29(5):1559–68. doi:10.1016/j.msec.2008.12.015.
Malda J, Woodfield TBF, Radisic M, Levenberg S, Oomens C, Baaijens FP, et al. Cell nutrition: in vitro and in vivo. Tissue engineering: a textbook. 2008. p. 327–62.
Friedrich HE. Magnesium technology: metallurgy, design data, applications. Heidelberg: Springer; 2006.
Sugawara M, Maeda N. Hemorheology and blood flow. Tokyo: Corona Publishing Co.; 2003.
Song G, Atrens A. Understanding magnesium corrosion: a framework for improved alloy performance. Adv Eng Mater. 2003;5(12):837–58.
Lee J-Y, Han G, Kim Y-C, Byun J-Y, Jang J-i, Seok H-K, et al. Effects of impurities on the biodegradation behavior of pure magnesium. Met Mater Int. 2009;15(6):955–61.
Montemor MF, Simões AM, Carmezim MJ. Characterization of rare-earth conversion films formed on the AZ31 magnesium alloy and its relation with corrosion protection. Appl Surf Sci. 2007;253(16):6922–31.
Gu X, Zheng Y, Zhong S, Xi T, Wang J, Wang W. Corrosion of, and cellular responses to Mg–Zn–Ca bulk metallic glasses. Biomaterials. 2010;31(6):1093–103.
Leon B, Jansen JA, editors. Thin calcium phosphate coatings for medical implants. New York: Springer; 2009.
Roberge PR. Handbook of corrosion engineering. New York: McGraw-Hill; 2000.
Regnier P, Lasaga AC, Berner RA, Han OH, Zilm KW. Mechanism of CO (super 2-) 3 substitution in carbonate-fluorapatite; evidence from FTIR spectroscopy, 13 C NMR, and quantum mechanical calculations. Am Mineral. 1994;79(9–10):809–18.
Rey C, Collins B, Goehl T, Dickson I, Glimcher M. The carbonate environment in bone mineral: a resolution-enhanced fourier transform infrared spectroscopy study. Calcif Tissue Int. 1989;45(3):157–64. doi:10.1007/bf02556059.
Tatzber M, Stemmer M, Spiegel H, Katzlberger C, Haberhauer G, Gerzabek M. An alternative method to measure carbonate in soils by FT-IR spectroscopy. Environ Chem Lett. 2007;5(1):9–12. doi:10.1007/s10311-006-0079-5.
Doi Y, Moriwaki Y, Aoba T, Takahashi J, Joshin K. ESR and IR studies of carbonate-containing hydroxyapatites. Calcif Tissue Int. 1982;34(1):178–81. doi:10.1007/bf02411230.
Burgess SK, Carey DM, Oxendine SL. Novel protein inhibits in vitro precipitation of calcium carbonate. Arch Biochem Biophys. 1992;297(2):383–7. doi:10.1016/0003-9861(92)90688-s.
Rettig R, Virtanen S. Time-dependent electrochemical characterization of the corrosion of a magnesium rare-earth alloy in simulated body fluids. J Biomed Mater Res A. 2008;85A(1):167–75.
Virtanen S. Corrosion of biomedical implant materials. Corros Rev. 2008;26(2):147–72.
Witte F, Kaese V, Haferkamp H, Switzer E, Meyer-Lindenberg A, Wirth CJ, et al. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials. 2005;26(17):3557–63.
Ren Y, Huang J, Zhang B, Yang K. Preliminary study of biodegradation of AZ31B magnesium alloy. Front Mater Sci China. 2007;1(4):401–4.
Ferguson JF, Jenkins D, Eastman J. Calcium phosphate precipitation at slightly alkaline pH values. J Water Pollut Control Fed. 1973;45(4):620–31.
Xin Y, Chu PK. Influence of Tris in simulated body fluid on degradation behavior of pure magnesium. Mater Chem Phys. 2010;124:33–5. doi:10.1016/j.matchemphys.2010.07.010.
Soares HMVM, Conde PCFL. Electrochemical investigations of the effect of N-substituted aminosulfonic acids with a piperazinic ring pH buffers on heavy metal processes which may have implications on speciation studies. Anal Chim Acta. 2000;421(1):103–11.
Sokolowska M, Bal W. Cu(II) complexation by “non-coordinating” N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES buffer). J Inorg Biochem. 2005;99(8):1653–60.
Song GL, Atrens A. Corrosion mechanisms of magnesium alloys. Adv Eng Mater. 1999;1:11–33.
Witte F, Nellesen J, Crostack H-A, Kaese V, Pisch A, Beckmann F, et al. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials. 2006;27(7):1013–8.
Li Z, Gu X, Lou S, Zheng Y. The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials. 2008;29(10):1329–44.
Wong HM, Yeung KWK, Lam KO, Tam V, Chu PK, Luk KDK, et al. A biodegradable polymer-based coating to control the performance of magnesium alloy orthopaedic implants. Biomaterials. 2010;31:2084–96.
Acknowledgments
The authors gratefully acknowledge financial support provided by the New Zealand Foundation for Research, Science and Technology (FRST). The authors would also like to thank Jemimah Walker for her input and assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nicholas T. Kirkland and Jay Waterman have contributed equally to this work
Rights and permissions
About this article
Cite this article
Kirkland, N.T., Waterman, J., Birbilis, N. et al. Buffer-regulated biocorrosion of pure magnesium. J Mater Sci: Mater Med 23, 283–291 (2012). https://doi.org/10.1007/s10856-011-4517-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-011-4517-y