Abstract
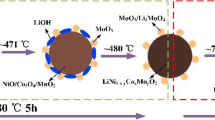
Structural instability and interface side reactions are considered to be the key factors that LiCoO2 (LCO) cannot maintain good electrochemical performance at voltages above 4.5 V. Here, a coating of lithium polyacrylate (LiPAA) was coated on the surface of the LCO by a wet chemical method. At a current density of 15 mA g−1, LCO coated with LiPAA coating (LCO@LiPAA) has a discharge capacity of 216.7 mAh g−1 in the voltage range of 2.7–4.6 V after 100 cycles, and the capacity retention rate is 84.53%. Even at a current density of 750 mA g−1, the LCO@LiPAA-positive electrode still has a discharge capacity of 130.3 mAh g−1. The results show that the uniform coating effectively blocks the side reactions between LCO and electrolyte, inhibits the occurrence of the irreversible phase transition of LCO, and improves the electrochemical performance of LCO@LiPAA cathode at 4.6 V.








Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
E. Hu, X. Yu, R. Lin, Evolution of redox couples in Li- and Mn-rich cathode materials and mitigation of voltage fade by reducing oxygen release. Nat. Energy 3, 690–698 (2018). https://doi.org/10.1038/s41560-018-0207-z
J. Zheng, Y. Han, D. Sun, In situ-formed LiVOPO4@V2O5 core-shell nanospheres as a cathode material for lithium-ion cell. Energy Storage Mater. 7, 48–55 (2017). https://doi.org/10.1016/j.ensm.2016.12.003
X. Yu, A. Manthiram, Electrode-electrolyte interfaces in lithium-based batteries. Energy Environ. Sci. 11, 527–543 (2018). https://doi.org/10.1039/C7EE02555F
J.I. Lee, E.H. Lee, J.H. Park, Ultrahigh-energy-density lithium-ion batteries based on a high-capacity anode and a high-voltage cathode with an electroconductive nanoparticle shell. Adv. Energy Mater. 4, 1301542 (2014). https://doi.org/10.1002/aenm.201301542
S. Kalluri, M. Yoon, M. Jo, Surface engineering strategies of layered LiCoO2 cathode material to realize high-energy and high-voltage Li-ion cells. Adv. Energy Mater. 7, 1601507 (2017). https://doi.org/10.1002/aenm.201601507
J.N. Reimers, J.R. Dahn, Electrochemical and in situ X-ray diffraction studies of lithium intercalation in LixCoO2. J. Electrochem. Soc. 139, 2091 (1992). https://doi.org/10.1149/1.2221184
L. Wang, B. Chen, J. Ma, Reviving lithium cobalt oxide-based lithium secondary batteries-toward a higher energy density. Chem. Soc. Rev. 47, 6505–6602 (2018). https://doi.org/10.1039/C8CS00322J
Z. Chen, J.R. Dahn, Methods to obtain excellent capacity retention in LiCoO2 cycled to 4.5 V. Electrochim. Acta 49, 1079–1090 (2004). https://doi.org/10.1016/j.electacta.2003.10.019
J.N. Zhang, Q.H. Li, C.Y. Ouyang, Trace doping of multiple elements enables stable battery cycling of LiCoO2 at 4.6 V. Nat. Energy 4, 594–603 (2019). https://doi.org/10.1038/s41560-019-0409-z
M.D. Radin, S. Hy, M. Sina, Narrowing the gap between theoretical and practical capacities in Li-ion layered oxide cathode materials. Adv. Energy Mater. 7, 1602888 (2017). https://doi.org/10.1002/aenm.201602888
H. Xia, L. Lu, Y.S. Meng, Phase transitions and high-voltage electrochemical behavior of LiCoO2 thin films grown by pulsed laser deposition. J. Electrochem. Soc. 154, 337–342 (2007). https://doi.org/10.1149/1.2509021
S.H. Min, M.R. Jo, S.Y. Choi, A layer-structured electrode material reformed by a PO4-O2 hybrid framework toward enhanced lithium storage and stability. Adv. Energy Mater. 6, 1501717 (2016). https://doi.org/10.1002/aenm.201501717
J.S. Park, A.U. Mane, J.W. Elam, J.R. Croy, Amorphous metal fluoride passivation coatings prepared by atomic layer deposition on LiCoO2 for Li-ion batteries. Chem. Mater. 27, 1917–1920 (2015). https://doi.org/10.1021/acs.chemmater.5b00603
Q. Liu, X. Su, D. Lei, Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping. Nat. Energy 3, 936–943 (2018). https://doi.org/10.1038/s41560-018-0180-6
N. Wu, Y. Zhang, Y. Wei, Template-engaged synthesis of 1D hierarchical chainlike LiCoO2 cathode materials with enhanced high-voltage lithium storage capabilities. ACS Appl. Mater. Interfaces 8, 25361–25368 (2016). https://doi.org/10.1021/acsami.6b09159
N. Wu, Y. Zhang, Y. Guo, S. Liu, H. Liu, Flake-like LiCoO2 with exposed 010 facets as a stable cathode material for highly reversible lithium storage. ACS Appl. Mater. Interfaces 8, 2723–2731 (2016). https://doi.org/10.1021/acsami.5b10977
N. Yabuuchi, Y. Kinoshita, K. Misaki, T. Matsuyama, Electrochemical properties of LiCoO2 electrodes with latex binders on high-voltage exposure. J. Electrochem. Soc. 162, A538–A544 (2015). https://doi.org/10.1149/2.0151504jes
Y.S. Jung, A.S. Cavanagh, L. Gedvilas, Improved functionality of lithium-ion batteries enabled by atomic layer deposition on the porous microstructure of polymer separators and coating electrodes. Adv. Energy Mater. 2, 1022–1027 (2012). https://doi.org/10.1002/aenm.201100750
H.S. Jeong, S.Y. Lee, Closely packed SiO2 nanoparticles/poly(vinylidene fluoride-hexafluoropropylene) layers-coated polyethylene separators for lithium-ion batteries. J. Power Sources 196, 6716–6722 (2011). https://doi.org/10.1016/j.jpowsour.2010.11.037
H.S. Jeong, D.W. Kim, Y.U. Jeong, Effect of phase inversion on microporous structure development of Al2O3/poly(vinylidene fluoride-hexafluoropropylene)-based ceramic composite separators for lithium-ion batteries. J. Power Sources 195, 6116–6121 (2010). https://doi.org/10.1016/j.jpowsour.2009.10.085
Y.M. Todorov, K. Fujii, N. Yoshimoto, Ion-solvation structure and battery electrode characteristics of nonflammable organic electrolytes based on tris(trifluoroethyl)phosphate dissolving lithium salts. Phys. Chem. Chem. Phys. 19, 31085 (2017). https://doi.org/10.1039/C7CP06438A
T.Q. Yong, L.Z. Zhang, J.L. Wang, Novel choline-based ionic liquids as safe electrolytes for high-voltage lithium-ion batteries. J. Power Sources 328, 397–404 (2016). https://doi.org/10.1016/j.jpowsour.2016.08.044
G. Alva, C. Kim, T. Yi, Surface chemistry consequences of Mg-based coatings on LiNi0.5Mn1.5O4 electrode materials upon operation at high voltage. J. Phys. Chem. C 118, 10596–10605 (2014). https://doi.org/10.1021/jp5003148
Z.Z. Wu, S.P. Ji, T.C. Liu, Y.D. Duan, Aligned Li+ tunnels in core-shell Li(NixMnyCoz)O2@LiFePO4 enhances its high voltage cycling stability as Li-ion battery cathode. Nano Lett. 16, 6357–6363 (2016). https://doi.org/10.1021/acs.nanolett.6b02742
M. Bettge, Y. Li, B. Sankaran, N.D. Rago, Improving high-capacity Li1.2Ni0.15Mn0.55Co0.1O2-based lithium-ion cells by modifiying the positive electrode with alumina. J. Power Sources 233, 346–357 (2013). https://doi.org/10.1016/j.jpowsour.2013.01.082
B. Shen, P.J. Zuo, Q. Li, X.S. He, Lithium cobalt oxides functionalized by conductive Al-doped ZnO coating as cathode for high-performance lithium ion batteries. Electrochim. Acta 224, 96–104 (2017). https://doi.org/10.1016/j.electacta.2016.12.037
L. Liang, G.R. Hu, F. Jiang, Y.B. Cao, Electrochemical behaviours of SiO2–coated LiNi0.8Co0.1Mn0.1O2 cathode materials by a novel modification method. J. Alloy. Compd. 657, 570–581 (2016). https://doi.org/10.1016/j.jallcom.2015.10.177
H. Kim, B. Park, S. Myung, Electrochemical and thermal characterization of AlF3-coated Li[Ni0.8Co0.15Al0.05]O2 cathode in lithium-ion cells. J. Power Sources 179, 347–350 (2008). https://doi.org/10.1016/j.jpowsour.2007.12.109
C.H. Jo, D.H. Cho, H.J. Noh, An effective method to reduce residual lithium compounds on Ni-rich Li[Ni0.6Co0.2Mn0.2]O2 active material using a phosphoric acid derived Li3PO4 nanolayer. Nano Res. 8, 1464–1479 (2015). https://doi.org/10.1007/s12274-014-0631-8
R. Gu, T. Cheng, Z.T. Ma, Enhanced cycling stability of high voltage LiCoO2 by surface phosphorylation. J. Alloy. Compd. 803, 348–353 (2019). https://doi.org/10.1016/j.jallcom.2019.06.253
G.Z. Lu, W.X. Peng, Y.T. Zhang, X.Q. Wang, X.X. Shi, Study on the formation, development and coating mechanism of new phases on interface in LiNbO3-coated LiCoO2. Electrochim. Acta 368, 137639 (2021). https://doi.org/10.1016/j.electacta.2020.137639
X. Wang, Q. Wu, S.Y. Li, Z.M. Tong, D. Wang, Lithium-Aluminum-Phosphate coating enables stable 4.6V cycling performance of LiCoO2 at room temperature and beyond. Energy Storage Mater. 37, 67–76 (2021). https://doi.org/10.1016/j.ensm.2021.01.031
L.Z. Huang, C.S. Kien, I. Ismail, Structural transformations of mechanically induced top-down approach BaFe12O19 nanoparticles synthesized from high crystallinity bulk materials. J. Magn. Magn. Mater. 429, 192–202 (2017). https://doi.org/10.1016/j.jmmm.2017.01.036
N.P.W. Pieczonka, V. Borgel, B. Ziv, N. Leifer, V. Dargel, Lithium polyacrylate (LiPAA) as an advanced binder and a passivating agent for high-voltage Li-ion batteries. Adv. Energy Mater. 5, 1501008 (2015). https://doi.org/10.1002/aenm.201501008
J. Lia, D.B. Le, P.P. Ferguson, Lithium polyacrylate as a binder for tin–cobalt–carbon negative electrodes in lithium-ion batteries. Electrochim. Acta 55, 2991–2995 (2010). https://doi.org/10.1016/j.electacta.2010.01.011
A. Virya, K. Lian, Lithium polyacrylate-polyacrylamide blend as polymer electrolytes for solidstate electrochemical capacitors. Electrochem. Commun. 97, 77–81 (2018). https://doi.org/10.1016/j.elecom.2018.10.026
Q. Zhang, J. Mei, X. Wang, High performance spinel LiNi0.5Mn1.5O4 cathode material by lithium polyacrylate coating for lithium ion battery. Electrochim. Acta 143, 265–271 (2014). https://doi.org/10.1016/j.electacta.2014.08.030
K. Mu, Y. Tao, Z. Peng, Surface architecture modification of high capacity Li1.2Ni0.2Mn0.6O2 with synergistic conductive polymers LiPPA and PPy for lithium ion batteries. Appl. Surf. Sci. 495, 143503 (2019). https://doi.org/10.1016/j.apsusc.2019.07.245
G.R. Hu, J. Fan, Y. Lu, Y.J. Zhang, K. Du, Surface architecture design of LiNi0.8Co0.15Al0.05O2 cathode with synergistic organics encapsulation to enhance electrochemical stability. Chemsuschem 13, 5699–5710 (2020). https://doi.org/10.1002/cssc.202001771
J.W. Qian, L. Liu, J.X. Yang, S.Y. Li, X. Wang, Electrochemical surface passivation of LiCoO2 particles at ultrahigh voltage and its applications in lithium-based batteries. Nat. Commun. 9, 4918–4928 (2018). https://doi.org/10.1038/s41467-018-07296-6
J.J. Zhang, J.H. Zhao, L.P. Yue, Safety-reinforced poly(propylene carbonate)-based all-solid-state polymer electrolyte for ambient-temperature solid polymer lithium batteries. Adv. Energy Mater. 5, 1501082 (2015). https://doi.org/10.1002/aenm.201501082
N.W. Li, Y. Shi, Y.X. Yin, Smart solid electrolyte interphase layer for long life lithium metal anodes. Angew. Chem. 57, 1505–1509 (2018). https://doi.org/10.1002/anie.201710806
SPd. Silva, L.E. Sita, CSd. Santos, J. Scarminio, Effects on the phases and crystalline structures of LiCoO2 cathode under thermal treatments up to 400°C. J. Alloy. Compd. 810, 151933–151940 (2019). https://doi.org/10.1016/j.jallcom.2019.151933
L.E. Sita, CSd. Santosb, SPd. Silva, A simple process to resynthesize the LiCoO2 and LiNi1/3Co1/3Mn1/3O2 compounds from the cathode material extracted from a batch of spent LCO batteries. J. Alloy. Compd. 894, 162350–162359 (2022). https://doi.org/10.1016/j.jallcom.2021.162350
F. Wu, M. Wang, Y.F. Su, A novel method for synthesis of layered LiNi1/3Mn1/3Co1/3O2 as cathode material for lithium-ion battery. J. Power Sources 195, 2362–2367 (2010). https://doi.org/10.1016/j.jpowsour.2009.10.043
Q. Wu, W.R. Li, Y. Cheng, Z.Y. Jiang, Homogenous LiCoO2 nanoparticles prepared using surfactant P123 as template and its application to manufacturing ultra-thin-film electrode. Mater. Chem. Phys. 91, 463–467 (2005). https://doi.org/10.1016/j.matchemphys.2004.12.011
L.L. Wang, J. Ma, C. Wang, X.R. Yu, R. Liu, A novel bifunctional self-stabilized strategy enabling 4.6V LiCoO2 with excellent long-term cyclability and high-rate capability. Adv. Sci. 6, 1900355–1900365 (2019). https://doi.org/10.1002/advs.201900355
C.F. Liu, C.K. Zhang, H.Q. Song, Mesocrystal MnO cubes as anode for Li-ion capacitors. Nano Energy 22, 290–300 (2016). https://doi.org/10.1016/j.nanoen.2016.02.035
J.-H. Park, J.-H. Cho, E.-H. Lee, Thickness-tunable polyimide nanoencapsulating layers and their influence on cell performance/thermal stability of high-voltage LiCoO2 cathode materials for lithium-ion batteries. J. Power Sources 244, 442–449 (2013). https://doi.org/10.1016/j.jpowsour.2012.11.111
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 52064035), the Key Research and Development Program of Gansu Province (21YF5GA078), and the Natural Science Foundation of Zhejiang Province (Grant No. LGG22E020003).
Funding
Funding were provided by National Natural Science Foundation of China (Grant No. 52064035), Key Research and Development Program of Gansu Province (Grant No. 21YF5GA078) and Natural Science Foundation of Zhejiang Province (Grant No. LGG22E020003).
Author information
Authors and Affiliations
Contributions
FZ and YM guided all the experimental design, and led the manuscript preparation and revision work. HG did most of the experiments, data analysis, and prepared the draft manuscript. XL conducted some experiments. All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All the authors do not have any possible conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, H., Meng, Y., Liu, X. et al. Improvement of electrochemical properties of LiCoO2 at 4.6 V by a LiPAA coating. J Mater Sci: Mater Electron 33, 17125–17136 (2022). https://doi.org/10.1007/s10854-022-08588-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08588-w