Abstract
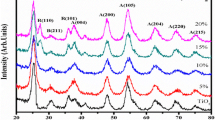
The primary objective of the present work is to analyse the effectiveness of lanthanum as a dopant material through sol–gel technique, onto pure γ-MnO2 (Nsutite) nanoparticles. The X-ray diffractograms confirm the structure of both pure and lanthanum doped samples, whilst bringing to light the drastic improvement in crystallite size (~ 10 nm) due to doping of lanthanum. The existence of various functional groups present in the titular material was affirmed using FTIR spectra. The multipoint BET surface area studies report a high surface area (101.03 m2/g) for pure samples and also discusses the variation with effect to doping of lanthanum. The morphological study done using HR-SEM on the pure γ-MnO2 samples show a rice-like morphology with a grain size of 20–35 nm. On the other hand, the lanthanum doped γ-MnO2 samples display a nano-twig morphology as seen in FE-SEM images. Elemental composition analysis was confirmed using EDX. The HR-TEM images clearly corroborate to the same morphology and the SAED pattern display a highly polycrystalline nature with a fringe spacing of d = 0.33 nm for pure γ-MnO2 samples and d = 0.43 nm for 1 mol% lanthanum doped samples. The SAED diffraction rings were indexed and they clearly support the XRD results. The magnetic study discusses the paramagnetic nature of the samples and also explains the large coercive force exhibited by the lanthanum doped samples.











Similar content being viewed by others
Data availability
The data used to support the finding of this work are included within the article.
References
R.N. Bhargava, Doped nanocrystalline materials—physics and applications. J. Lumin. 70(1–6), 85–94 (1996)
E.T. Goldburt, B. Kulkarni, R.N. Bhargava, J. Taylor, M. Libera, Size dependent efficiency in Tb doped Y2O3 nanocrystalline phosphor. J. Lumin. 72, 190–192 (1997)
R.W. Siegel, Nanostructured materials-mind over matter. Nanostruct. Mater. 3(1–6), 1–18 (1993)
E.F. Hilinski, P.A. Lucas, Y. Wang, A picosecond bleaching study of quantum-confined cadmium sulfide microcrystallites in a polymer film. J. Chem. Phys. 89(6), 3435–3441 (1988)
Y.Q. Chang, D.P. Yu, Y. Long, J. Xu, X.H. Luo, R.C. Ye, Large-scale fabrication of single-crystalline Mn3O4 nanowires via vapor phase growth. J. Cryst. Growth 279(1–2), 88–92 (2005)
B. Folch, J. Larionova, Y. Guari, C. Guérin, A. Mehdi, C. Reyé, Formation of Mn3O4 nanoparticles from the cluster [Mn12O12(C2H5 COO)16(H2O)3] anchored to hybrid mesoporous silica. J. Mater. Chem. 14(17), 2703–2711 (2004)
C.W. Na, D.S. Han, D.S. Kim, J. Park, Y.T. Jeon, G. Lee, M.H. Jung, Ferromagnetism of MnO and Mn3O4 nanowires. Appl. Phys. Lett. 87(14), 142504 (2005)
W.S. Seo, H.H. Jo, K. Lee, B. Kim, S.J. Oh, J.T. Park, Size-dependent magnetic properties of colloidal Mn3O4 and MnO nanoparticles. Angew. Chem. Int. Ed. 43(9), 1115–1117 (2004)
J. Park, K. An, Y. Hwang, J.G. Park, H.J. Noh, J.Y. Kim, J.H. Park, N.M. Hwang, T. Hyeon, Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 3(12), 891–895 (2004)
D. Zitoun, N. Pinna, N. Frolet, C. Belin, Single crystal manganese oxide multipods by oriented attachment. J. Am. Chem. Soc. 127(43), 15034–15035 (2005)
J. Jiang, A. Kucernak, Electrochemical supercapacitor material based on manganese oxide: preparation and characterization. Electrochim. Acta 47(15), 2381–2386 (2002)
Y. Chabre, J. Pannetier, Structural and electrochemical properties of the proton/γ-MnO2 system. Prog. Solid State Chem. 23(1), 1–130 (1995)
K. Suetsugu, T. Shoji, K. Sekitani, An investigation of structural water in electrolytic manganese dioxide (EMD). TOSOH Res. Technol. Rev. 49, 21 (2005)
D. Balachandran, D. Morgan, G. Ceder, First principles study of H-insertion in MnO2. J. Solid State Chem. 166(1), 91–103 (2002)
L.S. Dent Glasser, L. Ingram, Refinement of the crystal structure of groutite–MnOOH. Acta Crystallogr. Sect. B 24(9), 1233–1236 (1968)
C. Klingsberg, R. Roy, Stability and interconvertibility of phases in the system Mn-O-OH. Am. Mineral. 44(7–8), 819–838 (1959)
L.A.H. MacLean, F.L. Tye, The structure of fully H-inserted γ-manganese dioxide compounds. J. Solid State Chem. 123(1), 150–160 (1996)
M.H. Rossouw, D.C. Liles, M.M. Thackeray, W.I.F. David, S. Hull, Alpha manganese dioxide for lithium batteries: a structural and electrochemical study. Mater. Res. Bull. 27(2), 221–230 (1992)
J. Park, E. Kang, C.J. Bae, J.G. Park, H.J. Noh, J.Y. Kim, J.H. Park, H.M. Park, T. Hyeon, Synthesis, characterization, and magnetic properties of uniform-sized MnO nanospheres and nanorods. J. Phys. Chem. B 108(36), 13594–13598 (2004)
T. Ahmad, K.V. Ramanujachary, S.E. Lofland, A.K. Ganguli, Nanorods of manganese oxalate: a single source precursor to different manganese oxide nanoparticles (MnO, Mn2O3, Mn3O4). J. Mater. Chem. 14(23), 3406–3410 (2004)
C. Palache, H. Berman, C. Frondel, Dana’s System of Mineralogy, vol. II (The Georgia Mineral Society Inc, Norcross, 1951), pp. 439–442
J. Zhao, Z. Tao, J. Liang, J. Chen, Facile synthesis of nanoporous γ-MnO2 structures and their application in rechargeable Li-ion batteries. Cryst. Growth Des. 8(8), 2799–2805 (2008)
X.G. Zhang, C.M. Shen, H.L. Li, Preparation of γ-MnO2/carbon composite material by a wet chemical method. Mater. Res. Bull. 36(3–4), 541–546 (2001)
M. Behpour, A.M. Attaran, M.M. Sadiany, A. Khoobi, Adsorption effect of a cationic surfactant at carbon paste electrode as a sensitive sensor for study and detection of folic acid. Measurement 77, 257–264 (2016)
N.H. Arani, S.M. Ghoreishi, A. Khoobi, Increasing the electrochemical system performance using a magnetic nanostructured sensor for simultaneous determination of L-tyrosine and epinephrine. Anal. Methods 11(9), 1192–1198 (2019)
S.M. Ghoreishi, A. Khoobi, M. Behpour, S. Masoum, Application of multivariate curve resolution alternating least squares to biomedical analysis using electrochemical techniques at a nanostructure-based modified sensor. Electrochim. Acta 130, 271–278 (2014)
S.M. Ghoreishi, F.Z. Kashani, A. Khoobi, M. Enhessari, Fabrication of a nickel titanate nanoceramic modified electrode for electrochemical studies and detection of salicylic acid. J. Mol. Liq. 211, 970–980 (2015)
A.M. Hashem, H.M. Abuzeid, N. Narayanan, H. Ehrenberg, C.M. Julien, Synthesis, structure, magnetic, electrical and electrochemical properties of Al, Cu and Mg doped MnO2. Mater. Chem. Phys. 130(1–2), 33–38 (2011)
C.S. Sridhar, K.S.M. Laxmi, D.M. Potukuchi, C.S. Lakshmi, Dielectric properties of superparamagnetic titanium doped nanophased Mn–Zn ferrites for high frequency applications. Mater. Res. Express 6(12), 126117 (2020)
M.A. Almessiere, Y. Slimani, S. Rehman, F.A. Khan, Ç.D. Güngüneş, S. Güner, S.E. Shirsath, A. Baykal, Magnetic properties, anticancer and antibacterial effectiveness of sonochemically produced Ce3+/Dy3+ co-activated Mn-Zn nanospinel ferrites. Arab. J. Chem. 13(10), 7403–7417 (2020)
K. Tanbir, M.P. Ghosh, R.K. Singh, S. Mukherjee, Gd-doped soft Mn–Zn nanoferrites: synthesis, microstructural, magnetic and dielectric characterizations. J. Mater. Sci. 31(4), 3529–3538 (2020)
J. Fang, T. Liu, Z. Chen, Y. Wang, W. Wei, X. Yue, Z. Jiang, A wormhole-like porous carbon/magnetic particles composite as an efficient broadband electromagnetic wave absorber. Nanoscale 8(16), 8899–8909 (2016)
M. Kaiser, Effect of rare earth elements on the structural, magnetic and electrical behavior of Ni-Zn-Cr nanoferrites. J. Alloy. Compd. 719, 446–454 (2017)
S. Jauhar, S. Singhal, Chromium and copper substituted lanthanum nano-ferrites: their synthesis, characterization and application studies. J. Mol. Struct. 1075, 534–541 (2014)
G. Shao, Y. Yao, S. Zhang, P. He, Supercapacitor characteristic of La-doped Ni(OH)2 prepared by electrode-position. Rare Met. 28(2), 132–136 (2009)
R.V. Wandekar, B.N. Wani, S.R. Bharadwaj, High temperature thermal expansion and electrical conductivity of Ln0.95MnO3+δ (Ln= La, Nd or Gd). J. Alloy. Compd. 433(1–2), 84–90 (2007)
M.K. Bharti, S. Chalia, P. Thakur, A. Thakur, Effect of lanthanum doping on microstructural, dielectric and magnetic properties of Mn0.4Zn0.6Cd0.2LaxFe1.8-xO4 (0.0≤ x≤ 0.4). J. Supercond. Novel Magn. 2021, 1–10 (2021)
R.R. Kanna, N. Lenin, K. Sakthipandi, M. Sivabharathy, Impact of lanthanum on structural, optical, dielectric and magnetic properties of Mn1-xCuxFe185La015O4 spinel nanoferrites. Ceram. Int 43(17), 15868–15879 (2017)
V.J. Mane, D.B. Malavekar, S.B. Ubale, R.N. Bulakhe, I.N. Insik, C.D. Lokhande, Binder free lanthanum doped manganese oxide@ graphene oxide composite as high energy density electrode material for flexible symmetric solid state supercapacitor. Electrochim. Acta 335, 135613 (2020)
N. Lenin, R. Rajesh Kanna, K. Sakthipandi, A. Senthil Kumar, Structural, electrical and magnetic properties of NiLaxFe2-xO4 nanoferrites. Mater. Chem. Phys. 212, 385–393 (2018)
K. Chen, W. Pan, D. Xue, Phase transformation of Ce3+- doped MnO2 for pseudocapacitive electrode materials. J. Phys. Chem. C 120(36), 20077–20081 (2016)
R. Rajagopal, K.-S. Ryu, Synthesis of La and Ce mixed MnO2 nanostructure/rGO composite for supercapacitor applications. ChemElectroChem 5(16), 2218–2227 (2018)
X.R. Jing-Wang, T.Y. Bao-Lian, Lanthanum doped manganese dioxide/carbon nanotube composite electrodes for electrochemical supercapacitors. Acta Phys. Chim. Sin. 27(10), 2340–2346 (2011)
A.A. Yadav, V.S. Kumbhar, S.J. Patil, N.R. Chodankar, C.D. Lokhande, Supercapacitive properties of chemically deposited La2O3 thin film. Ceram. Int. 42(1), 2079–2084 (2016)
X. Liu, C. Chen, Y. Zhao, B. Jia, A review on the synthesis of manganese oxide nanomaterials and their applications on lithium-ion batteries. J. Nanomater. 2013, 736375 (2013)
M. Shaban, A.M. El Sayed, Effects of lanthanum and sodium on the structural, optical and hydrophilic properties of sol–gel derived ZnO films: A comparative study. Mater. Sci. Semicond. Process. 41, 323–334 (2016)
M.V. Ananth, S. Pethkar, K. Dakshinamurthi, Distortion of MnO6 octahedra and electrochemical activity of Nstutite-based MnO2 polymorphs for alkaline electrolytes—an FTIR study. J. Power Sources 75(2), 278–282 (1998)
F.R. Mariosi, J. Venturini, A. da Cas Viegas, C.P. Bergmann, Lanthanum-doped spinel cobalt ferrite (CoFe2O4) nanoparticles for environmental applications. Ceram. Int. 46(3), 2772–2779 (2020)
M.S. Hamdy, B.M. Al-Shehri, K.S. Al-Namshah, M. Shkir, Synthesis, characterization, and photoluminescence property of Nd–TUD-1. Luminescence 36(1), 192–199 (2021)
B.M. Al-Shehri, T.M. Bawazeer, M.S. Alsoufi, M. Shkir, M.S. Hamdy, Facile synthesis, characterization, and photoluminescence property of lanthanum incorporated TUD-1. Optik 241, 166925 (2021)
M.A. Ratner, D. Ratner, Nanotechnology: A Gentle Introduction to the Next Big Idea (Prentice Hall Professional, Hoboken, 2003)
J. Lai, K.V. Shafi, A. Ulman, K. Loos, N.L. Yang, M.H. Cui, T. Vogt, C. Estournès, D.C. Locke, Mixed iron− manganese oxide nanoparticles. J. Phys. Chem. B 108(39), 14876–14883 (2004)
R. Saravanan, S. Francis, J. Berchmans, Doping level of Mn in high temperature grown Zn1−xMnxO studied through electronic charge distribution, magnetization, and local structure. Chem. Pap. 66(3), 226–234 (2012)
K. Ali, A. Bahadur, A. Jabbar, S. Iqbal, I. Ahmad, M.I. Bashir, Synthesis, structural, dielectric and magnetic properties of CuFe2O4/MnO2 nanocomposites. J. Magn. Magn. Mater. 434, 30–36 (2017)
S. Saravanakumar, S. Sasikumar, S. Israel, G.R. Pradhiba, R. Saravanan, Structural, magnetic and charge-related properties of nano-sized cerium manganese oxide, a dilute magnetic oxide semiconductor. Mater. Sci. Semicond. Process. 17, 186–193 (2014)
T. Abe, T. Kisi, A. Yasumori, Magnetic properties of glass ceramic in Fe3O4-MnO2-SiO2 system. J. Phys. 232(1), 012018 (2010)
A. Aslinjensipriya, R. Sylvia Reena, R. Ragu, S. Grace Infantiya, G. Mangalam, C. Justin Raj, S. Jerome Das, Exploring the influence of tin in micro-structural, magneto-optical and antimicrobial traits of nickel oxide nanoparticles. Surf. Interfaces 2021, 101605 (2021)
J. Al Boukhari, L. Zeidan, A. Khalaf, R. Awad, Synthesis, characterization, optical and magnetic properties of pure and Mn, Fe and Zn doped NiO nanoparticles. Chem. Phys. 516, 116–124 (2019)
Acknowledgements
The author records her thanks to the various Instrumentation facilities extended by IIT-M (SAIF), IISc., (SATF), Bangalore, Department of Nuclear Physics, Madras University and NCNSNT, Madras University.
Author information
Authors and Affiliations
Contributions
SAJ—conceptualization, investigation, writing original draft. RR—visualization, software validation, formal analysis. MMJ—validation. AD—validation, conceptualization. SJD—overall mentorship for this research activity.
Corresponding author
Ethics declarations
Conflict of interest
All authors of this manuscript have expressed their consent to send the manuscript in the current revised form and they assure that there are no conflict of interest.
Ethical approval
The authors assure, all the experiments and studies carried out in current study obey the standards ethics of the research committees, both international and national. Also, they declare that the submission is an exclusive to "Journal of Materials Science: Materials in Electronics (JMSE)" and not under consideration for publication elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jacob, S.A., Ragu, R., Jaculine, M.M. et al. Exploring the consequences of lanthanum incorporation on micro-structural, nanoscale morphological and magnetic traits on manganese dioxide nanoparticles. J Mater Sci: Mater Electron 33, 6856–6871 (2022). https://doi.org/10.1007/s10854-022-07863-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-07863-0



