Abstract
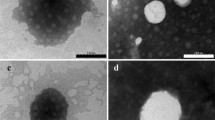
The objective of this study was to develop and characterize a novel combined systems of amphotericin B-loaded silk fibroin nanoparticles (AmB-FNPs) and in situ hydrogel for ocular administration. Three different formulations of AmB-FNPs were successfully prepared, by desolvation method, using polyethylenimine (PEI) or polyethylene glycol (PEG) as a coating polymer. All AmB-FNPs exhibited mean size of ~ 200 nm with narrow size distribution. The uncoated AmB-FNP and AmB-FNP-PEG were spherical in shape with zeta potential of ~ − 23 mV, whereas AmB-FNP-PEI exhibited cubic shape with zeta potential ~ + 36 mV. AmB was entrapped in FNPs in a partial aggregated form of AmB, which could reduce eye irritation compared to the marketed AmB deoxycholate. Then, AmB-FNPs were incorporated into two optimal thermosensitive in situ hydrogels; pluronic F127 (F127), and F127 and hyaluronic acid (F127/HA). All AmB-FNPs-in situ hydrogels exhibited homogeneous solutions with translucent light–yellow color, pH ~ 7, and osmolality of ~ 320–370 mOsmo/kg. At the ocular temperature, 35 °C, they manifested a pseudoplastic flow behavior and showed a rapid sol–gel transition within 30 s. In addition, the in vitro drug release studies showed an initial AmB burst release within 5 min. Finally, sterility test confirmed no microbial growth of all formulations. Overall results indicated that AmB-FNPs-in situ hydrogels showed satisfactory physicochemical properties as an ophthalmic formulations, which would reduce toxicity of AmB, increase precorneal residence time and improve patient compliance due to less frequent administration.






Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Ezisi CN, Ogbonnaya CE, Okoye O, Ezeanosike E, Ginger-Eke H, Arinze OC (2018) Microbial keratitis-a review of epidemiology, pathogenesis, ocular manifestations, and management. Niger J Ophthalmol 26:13–23
Mahmoudi S, Masoomi A, Ahmadikia K, Tabatabaei SA, Soleimani M, Rezaie S, Ghahvechian H, Banafsheafshan A (2018) Fungal keratitis: an overview of clinical and laboratory aspects. Mycoses 61(12):916–930. https://doi.org/10.1111/myc.12822
Ansari Z, Miller D, Galor A (2013) Current thoughts in fungal keratitis: diagnosis and treatment. Curr Fungal Infect Rep 7(3):209–218. https://doi.org/10.1007/s12281-013-0150-110.1007/s12281-013-0150-1
Austin A, Lietman T, Rose-Nussbaumer J (2017) Update on the management of infectious keratitis. Ophthalmology 124(11):1678–1689. https://doi.org/10.1016/j.ophtha.2017.05.012
Cavassin FB, Baú-Carneiro JL, Vilas-Boas RR, Queiroz-Telles F (2021) Sixty years of amphotericin B: an overview of the main antifungal agent used to treat invasive fungal infections. Infect Dis Ther 10(1):115–147. https://doi.org/10.1007/s40121-020-00382-7
Chhonker YS, Prasad YD, Chandasana H, Vishvkarma A, Mitra K, Shukla PK, Bhatta RS (2015) Amphotericin-B entrapped lecithin/chitosan nanoparticles for prolonged ocular application. Int J Biol Macromol 72:1451–1458. https://doi.org/10.1016/j.ijbiomac.2014.10.014
Serrano DR, Ruiz-Saldaña HK, Molero G, Ballesteros MP, Torrado JJ (2012) A novel formulation of solubilised amphotericin B designed for ophthalmic use. Int J Pharm 437(1–2):80–82. https://doi.org/10.1016/j.ijpharm.2012.07.065
Sharma A, Taniguchi J (2017) Review: emerging strategies for antimicrobial drug delivery to the ocular surface: Implications for infectious keratitis. Ocul Surf 15(4):670–679. https://doi.org/10.1016/j.jtos.2017.06.001
Hartsel SC, Bauer E, Kwong EH, Wasan KM (2001) The effect of serum albumin on amphotericin B aggregate structure and activity. Pharm Res 18(9):1305–1309. https://doi.org/10.1023/a:1013090011952
Adler-Moore JP, Proffitt RT (2008) Amphotericin B lipid preparations: what are the differences? Clin Microbiol Infect 14(Suppl 4):25–36. https://doi.org/10.1111/j.1469-0691.2008.01979.x
Patel A, Cholkar K, Agrahari V, Mitra AK (2013) Ocular drug delivery systems: an overview. World J Pharmacol 2(2):47–64. https://doi.org/10.5497/wjp.v2.i2.47
Tsai CH, Wang PY, Lin IC, Huang H, Liu GS, Tseng CL (2018) Ocular drug delivery: role of degradable polymeric nanocarriers for ophthalmic application. Int J Mol Sci 19(9):2830. https://doi.org/10.3390/ijms19092830
Destruel P-L, Zeng N, Maury M, Mignet N, Boudy V (2017) In vitro and in vivo evaluation of in situ gelling systems for sustained topical ophthalmic delivery: state of the art and beyond. Drug Discov 22(4):638–651. https://doi.org/10.1016/j.drudis.2016.12.008
Das S, Suresh PK (2011) Nanosuspension: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to amphotericin B. Nanomedicine: Nanotechnol Biol Med 7(2):242–247. https://doi.org/10.1016/j.nano.2010.07.003
Fu T, Yi J, Lv S, Zhang B (2017) Ocular amphotericin B delivery by chitosan-modified nanostructured lipid carriers for fungal keratitis-targeted therapy. J Liposome Res 27(3):228–233. https://doi.org/10.1080/08982104.2016.1224899
Almeida H, Amaral MH, Lobão P, Lobo JMS (2014) In situ gelling systems: a strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov 19(4):400–412. https://doi.org/10.1016/j.drudis.2013.10.001
Yang P, Dong Y, Huang D, Zhu C, Liu H, Pan X, Wu C (2019) Silk fibroin nanoparticles for enhanced bio-macromolecule delivery to the retina. Pharm Dev Technol 24(5):575–583. https://doi.org/10.1080/10837450.2018.1545236
Pham DT, Tiyaboonchai W (2020) Fibroin nanoparticles: a promising drug delivery system. Drug Deliv 27(1):431–448. https://doi.org/10.1080/10717544.2020.1736208
Chomchalao P, Nimtrakul P, Pham DT, Tiyaboonchai W (2020) Development of amphotericin B-loaded fibroin nanoparticles: a novel approach for topical ocular application. J Mater Sci 55(12):5268–5279. https://doi.org/10.1007/s10853-020-04350-x
Nikogeorgos N, Patil NJ, Zappone B, Lee S (2016) Interaction of porcine gastric mucin with various polycations and its influence on the boundary lubrication properties. Polymer 100:158–168. https://doi.org/10.1016/j.polymer.2016.08.030
Suk JS, Xu Q, Kim N, Hanes J, Ensign LM (2016) PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev 99:28–51. https://doi.org/10.1016/j.addr.2015.09.012
Lynch C, Kondiah PPD, Choonara YE, du Toit LC, Ally N, Pillay V (2019) Advances in biodegradable nano-sized polymer-based ocular drug delivery. Polymers 11(8):1371. https://doi.org/10.3390/polym11081371
Zhang K, Shi X, Lin X, Yao C, Shen L, Feng Y (2015) Poloxamer-based in situ hydrogels for controlled delivery of hydrophilic macromolecules after intramuscular injection in rats. Drug Deliv 22(3):375–382. https://doi.org/10.3109/10717544.2014.891272
Al Khateb K, Ozhmukhametova EK, Mussin MN, Seilkhanov SK, Rakhypbekov TK, Lau WM, Khutoryanskiy VV (2016) In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int J Pharm 502(1):70–79. https://doi.org/10.1016/j.ijpharm.2016.02.027
Pham DT, Saelim N, Tiyaboonchai W (2018) Crosslinked fibroin nanoparticles using EDC or PEI for drug delivery: physicochemical properties, crystallinity and structure. J Mater Sci 53(20):14087–14103
Lai C-F, Li J-S, Fang Y-T, Chien C-J, Lee C-H (2018) UV and blue-light anti-reflective structurally colored contact lenses based on a copolymer hydrogel with amorphous array nanostructures. RSC Adv 8(8):4006–4013. https://doi.org/10.1039/c7ra12753g
Baranowski P, Karolewicz B, Gajda M, Pluta J (2014) Ophthalmic drug dosage forms: characterisation and research methods. Sci World J 2014:14. https://doi.org/10.1155/2014/861904
Almeida H, Amaral MH, Lobao P, Silva AC, Loboa JM (2014) Applications of polymeric and lipid nanoparticles in ophthalmic pharmaceutical formulations: present and future considerations. J Pharm Pharm Sci 17(3):278–293. https://doi.org/10.18433/j3dp43
Pham DT, Saelim N, Tiyaboonchai W (2019) Alpha mangostin loaded crosslinked silk fibroin-based nanoparticles for cancer chemotherapy. Coll Surf B 181:705–713. https://doi.org/10.1016/j.colsurfb.2019.06.011
Bhat NV, Nadiger GS (1980) Crystallinity in silk fibers: partial acid hydrolysis and related studies. J Appl Polym Sci 25(5):921–932. https://doi.org/10.1002/app.1980.070250518
Irimia T, Ghica MV, Popa L, Anuţa V, Arsene A-L, Dinu-Pîrvu C-E (2018) Strategies for improving ocular drug bioavailability and corneal wound healing with chitosan-based delivery systems. Polymers 10(11):1221. https://doi.org/10.3390/polym10111221
Thoniyot P, Tan MJ, Karim AA, Young DJ, Loh XJ (2015) Nanoparticle-hydrogel composites: concept, design, and applications of these promising. Multi-Funct Mater Adv Sci 2(1–2):1400010. https://doi.org/10.1002/advs.201400010
Argenta DbF, Santos TCd, Campos AM, Caon T (2019) Hydrogel nanocomposite systems: physicochemical characterization and application for drug-delivery systems, in nanocarriers for drug delivery: Nanoscience and nanotechnology in drug delivery. Elsevier In. 81–131
Cabana A, Aït-Kadi A, Juhász J (1997) Study of the Gelation Process of Polyethylene Oxidea-Polypropylene Oxideb–Polyethylene OxideaCopolymer (Poloxamer 407) Aqueous Solutions. J Colloid Interface Sci 190(2):307–312. https://doi.org/10.1006/jcis.1997.4880
Dumortier G, Grossiord JL, Agnely F, Chaumeil JC (2006) A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res 23(12):2709–2728. https://doi.org/10.1007/s11095-006-9104-4
Ruel-Gariépy E, Leroux J-C (2004) In situ-forming hydrogels—review of temperature-sensitive systems. Eur J Pharm Biopharm 58(2):409–426. https://doi.org/10.1016/j.ejpb.2004.03.019
Jung Y-s, Park W, Park H, Lee D-K, Na K (2017) Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr Polym 156(Supplement C) 156:403–408. https://doi.org/10.1016/j.carbpol.2016.08.068
Choi SW, Kim J (2018) Therapeutic contact lenses with polymeric vehicles for ocular drug delivery: a review. Materials (Basel) 11(7):1125. https://doi.org/10.3390/ma11071125
Lanier OL, Manfre MG, Bailey C, Liu Z, Sparks Z, Kulkarni S, Chauhan A (2021) Review of approaches for increasing ophthalmic bioavailability for eye drop formulations. AAPS PharmSciTech 22(3):1–16. https://doi.org/10.1208/s12249-021-01977-0
Kumar D, Jain N, Gulati N, Nagaich U (2013) Nanoparticles laden in situ gelling system for ocular drug targeting. J Adv Pharm Technol Res 4(1):9–17. https://doi.org/10.4103/2231-4040.107495
Jain D, Kumar V, Singh S, Mullertz A, Bar-Shalom D (2016) Newer trends in in situ gelling systems for controlled ocular drug delivery. J Anal Pharm Res 2(3):1–16
El-Kamel AH (2002) In vitro and in vivo evaluation of Pluronic F127-based ocular delivery system for timolol maleate. Int J Pharm 241(1):47–55. https://doi.org/10.1016/S0378-5173(02)00234-X
Morsi N, Ghorab D, Refai H, Teba H (2016) Ketoroloac tromethamine loaded nanodispersion incorporated into thermosensitive in situ gel for prolonged ocular delivery. Int J Pharm 506(1):57–67. https://doi.org/10.1016/j.ijpharm.2016.04.021
Wu C, Qi H, Chen W, Huang C, Su CC, Li W, Hou S (2007) Preparation and evaluation of a Carbopol/HPMC-based in situ gelling ophthalmic system for puerarin. Yakugaku Zasshi 127(1):183–191
Shivam UU, Siddhi KC, Devarshi UG, Umeshkumar MU, Jayvadan KP (2020) Nanoparticles laden In situ gel for sustained drug release after topical ocular administration. J Drug Deliv Sci Technol 57:101736. https://doi.org/10.1016/j.jddst.2020.101736
Singh J, Chhabra G, Pathak K (2014) Development of acetazolamide-loaded, pH-triggered polymeric nanoparticulate in situ gel for sustained ocular delivery: in vitro. ex vivo evaluation and pharmacodynamic study. Drug Dev Ind Pharm 40:1223–1232
Barwicz J, Christian S, Gruda I (1992) Effects of the aggregation state of amphotericin B on its toxicity to mice. Antimicrob Agents Chemother 36(10):2310–2315. https://doi.org/10.1128/aac.36.10.2310
Churchill DN, Seely J (1977) Nephrotoxicity associated with combined gentamicin-amphotericin B therapy. Nephron 19(3):176–181. https://doi.org/10.1159/000180883
Adams ML, Kwon GS (2003) Relative aggregation state and hemolytic activity of amphotericin B encapsulated by poly(ethylene oxide)-block–poly(N-hexyl-l-aspartamide)-acyl conjugate micelles: effects of acyl chain length. J Control Release 87(1):23–32. https://doi.org/10.1016/S0168-3659(02)00347-4
Barwicz J, Tancrède P (1997) The effect of aggregation state of amphotericin-B on its interactions with cholesterol-or ergosterol-containing phosphatidylcholine monolayers. Chem Phys Lipids 85(2):145–155. https://doi.org/10.1016/S0009-3084(96)02652-7
Zia Q, Khan AA, Swaleha Z, Owais M (2015) Self-assembled amphotericin B-loaded polyglutamic acid nanoparticles: preparation, characterization and in vitro potential against Candida albicans. Int J Nanomedicine 10:1769–1790. https://doi.org/10.2147/ijn.s63155
Pham DT, Saelim N, Tiyaboonchai W (2019) Alpha mangostin loaded crosslinked silk fibroin-based nanoparticles for cancer chemotherapy. Coll Surf B Biointerfaces 181:705–713. https://doi.org/10.1016/j.colsurfb.2019.06.011
Acknowledgements
This research was supported by the National Research Council of Thailand (NRCT) under the Royal Golden Jubilee Ph.D. program [Grant No. PHD/0160/2560]. The authors are thankful to Faculty of Pharmaceutical Sciences, Naresuan University for providing necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chomchalao, P., Saelim, N. & Tiyaboonchai, W. Preparation and characterization of amphotericin B-loaded silk fibroin nanoparticles-in situ hydrogel composites for topical ophthalmic application. J Mater Sci 57, 12522–12539 (2022). https://doi.org/10.1007/s10853-022-07413-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07413-3