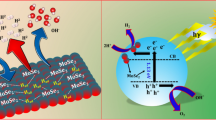
Abstract
A facile two-step hydrothermal approach is adopted to synthesize MoSe2/N-doped RGO (NG) composites with the N/C atomic percentage changing from 1.13 to 5.16 at%. In the composites, nanoclusters of MoSe2 nanosheets are dispersed on plicated NG nanosheets. The electrochemical measurement suggests that the MoSe2/NG composites exhibit enhanced electro-catalytic HER activity as compared to MoSe2 and MoSe2/RGO. Moreover, as the N/C ratio of NG is increased, the activity of MoSe2/NG increases firstly and then decreases. At low N/C ratio, the impact of interfacial energy barrier between MoSe2 and NG is negligible and the electron transfer is substantial, so the activity of the MoSe2/NG composites increases with carrier concentration in NG. However, at high N/C ratio, the energy barrier blocks the electron transfer from NG to MoSe2 remarkably. Consequently, the MoSe2/NG composites with an intermediate N/C ratio have the highest activity. Owing to the synergistic effect of NG and MoSe2, the Tafel slope of the composites is reduced from 114.69 to 78.45 mV dec−1 by 32% as compared to pure MoSe2. The results provide us valuable information for efficient design of transition metal dichalcogenide catalysts for electro-catalytic hydrogen evolution.








Similar content being viewed by others
References
Zhuang M, Ou X, Dou Y et al (2016) Polymer-embedded fabrication of Co2P nanoparticles encapsulated in N, P-doped graphene for hydrogen generation. Nano Lett 16:4691–4698. doi:10.1021/acs.nanolett.6b02203
Xie J, Zhang H, Li S et al (2013) Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv Mater 25:5807–5813. doi:10.1002/adma.201302685
An L, Huang L, Zhou P, Yin J, Liu H, Xi P (2015) A self-standing high-performance hydrogen evolution electrode with nanostructured NiCo2O4/CuS heterostructures. Adv Funct Mater 25:6814–6822. doi:10.1002/adfm.201503784
Zhou W, Lu J, Zhou K et al (2016) CoSe2 nanoparticles embedded defective carbon nanotubes derived from MOFs as efficient electrocatalyst for hydrogen evolution reaction. Nano Energy 28:143–150. doi:10.1016/j.nanoen.2016.08.040
Zhong X, Sun Y, Chen X, Zhuang G, Li X, Wang J-G (2016) Mo doping induced more active sites in urchin-like W18O49 nanostructure with remarkably enhanced performance for hydrogen evolution reaction. Adv Funct Mater 26:5778–5786. doi:10.1002/adfm.201601732
Dai X, Li Z, Du K et al (2015) Facile synthesis of in–situ nitrogenated graphene decorated by few-layer MoS2 for hydrogen evolution reaction. Electrochim Acta 171:72–80. doi:10.1016/j.electacta.2015.05.017
Kong D, Wang H, Lu Z, Cui Y (2014) CoSe2 nanoparticles grown on carbon fiber paper: an efficient and stable electrocatalyst for hydrogen evolution reaction. JACS 136:4897–4900. doi:10.1021/ja501497n
Zhang Y, Zuo L, Zhang L et al (2016) Cotton wool derived carbon fiber aerogel supported few-layered MoSe2 nanosheets as efficient electrocatalysts for hydrogen evolution. ACS Appl Mater Interfaces 8:7077–7085. doi:10.1021/acsami.5b12772
Zhang Y, Chang TR, Zhou B et al (2014) Direct observation of the transition from indirect to direct bandgap in atomically thin epitaxial MoSe2. Nat Nanotechnol 9:111–115. doi:10.1038/nnano.2013.277
Tang H, Dou KP, Kaun CC, Kuang Q, Yang SH (2014) MoSe2 nanosheets and their graphene hybrids: synthesis, characterization and hydrogen evolution reaction studies. J Mater Chem A 2:360–364. doi:10.1039/C3ta13584e
Larentis S, Fallahazad B, Tutuc E (2012) Field-effect transistors and intrinsic mobility in ultra-thin MoSe2 layers. Appl Phys Lett 101:223104. doi:10.1063/1.4768218
Xia J, Huang X, Liu L-Z et al (2014) CVD synthesis of large-area, highly crystalline MoSe2 atomic layers on diverse substrates and application to photodetectors. Nanoscale 6:8949–8955. doi:10.1039/c4nr02311k
Shim GW, Yoo K, Seo S-B et al (2014) Large-area single-layer MoSe2 and its van der Waals heterostructures. ACS Nano 8:6655–6662. doi:10.1021/nn405685j
Lei Z, Xu S, Wu P (2016) Ultra-thin and porous MoSe2 nanosheets: facile preparation and enhanced electrocatalytic activity towards the hydrogen evolution reaction. PCCP 18:70–74
Xu C, Peng S, Tan C et al (2014) Ultrathin S-doped MoSe2 nanosheets for efficient hydrogen evolution. J Mater Chem A 2:5597–5601. doi:10.1039/c4ta00458b
Gong Q, Cheng L, Liu C et al (2015) Ultrathin MoS2(1–x )Se2x alloy nanoflakes for electrocatalytic hydrogen evolution reaction. ACS Catal 5:2213–2219. doi:10.1021/cs501970w
Gupta U, Naidu BS, Maitra U et al (2014) Characterization of few-layer 1T-MoSe2 and its superior performance in the visible-light induced hydrogen evolution reaction. APL Mater 2:092802. doi:10.1063/1.4892976
Yuan HY, Li JY, Yuan C, He Z (2014) Facile synthesis of MoS2@CNT as an effective catalyst for hydrogen production in microbial electrolysis cells. Chemelectrochem 1:1828–1833. doi:10.1002/celc.201402150
Liao L, Zhu J, Bian X et al (2013) MoS2 formed on mesoporous graphene as a highly active catalyst for hydrogen evolution. Adv Funct Mater 23:5326–5333. doi:10.1002/adfm.201300318
Gopalakrishnan K, Pramoda K, Maitra U, Mahima U, Shah MA, Rao CNR (2015) Performance of MoS2-reduced graphene oxide nanocomposites in supercapacitors and in oxygen reduction reaction. Nanomater Energy 4:9–17. doi:10.1680/nme.14.00024
Ma C-B, Qi X, Chen B et al (2014) MoS2 nanoflower-decorated reduced graphene oxide paper for high-performance hydrogen evolution reaction. Nanoscale 6:5624–5629
Zhang Z, Lu B, Hao J, Yang W, Tang J (2014) FeP nanoparticles grown on graphene sheets as highly active non-precious-metal electrocatalysts for hydrogen evolution reaction. Chem Commun 50:11554–11557
Pramoda K, Gupta U, Ahmad I, Kumar R, Rao CNR (2016) Assemblies of covalently cross-linked nanosheets of MoS2 and of MoS2–RGO: synthesis and novel properties. J Mater Chem A 4:8989–8994. doi:10.1039/c6ta00645k
Zhong J, Deng J-J, Mao B-H et al (2012) Probing solid state N-doping in graphene by X-ray absorption near-edge structure spectroscopy. Carbon 50:335–338. doi:10.1016/j.carbon.2011.08.046
Pan Y, Yang N, Chen Y et al (2015) Nickel phosphide nanoparticles-nitrogen-doped graphene hybrid as an efficient catalyst for enhanced hydrogen evolution activity. J Power Sources 297:45–52. doi:10.1016/j.jpowsour.2015.07.077
Gopalakrishnan K, Rao CNR (2015) Remarkable performance of heavily nitrogenated graphene in the oxygen reduction reaction of fuel cells in alkaline medium. Mater Res Exp 2:095503. doi:10.1088/2053-1591/2/9/095503
Jia L, Wang D-H, Huang Y-X, Xu A-W, Yu H-Q (2011) Highly durable N-Doped graphene/CdS nanocomposites with enhanced photocatalytic hydrogen evolution from water under visible light irradiation. J Phys Chem C 115:11466–11473. doi:10.1021/jp2023617
Huang Z, Lv C, Chen Z, Chen Z, Tian F, Zhang C (2015) One-pot synthesis of diiron phosphide/nitrogen-doped graphene nanocomposite for effective hydrogen generation. Nano Energy 12:666–674
Zhao K, Gu W, Zhao L, Zhang C, Peng W, Xian Y (2015) MoS2/Nitrogen-doped graphene as efficient electrocatalyst for oxygen reduction reaction. Electrochim Acta 169:142–149. doi:10.1016/j.electacta.2015.04.044
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. JACS 80:1339. doi:10.1021/ja01539a017
Du C, Huang H, Feng X, Wu S, Song W (2015) Confining MoS2 nanodots in 3D porous nitrogen-doped graphene with amendable ORR performance. J Mater Chem A 3:7616–7622. doi:10.1039/c5ta00648a
Ma J, Habrioux A, Luo Y et al (2015) Electronic interaction between platinum nanoparticles and nitrogen-doped reduced graphene oxide: effect on the oxygen reduction reaction. J Mater Chem A 3:11891–11904. doi:10.1039/c5ta01285f
Mao S, Wen ZH, Ci SQ, Guo XR, Ostrikov K, Chen JH (2015) Perpendicularly oriented MoSe2/graphene nanosheets as advanced electrocatalysts for hydrogen evolution. Small 11:414–419. doi:10.1002/smll.201401598
Chang K, Mei Z, Wang T, Kang Q, Ouyang S, Ye J (2014) MoS2/graphene cocatalyst for efficient photocatalytic H2 evolution under visible light irradiation. ACS Nano 8:7078–7087. doi:10.1021/nn5019945
Kong DS, Wang HT, Cha JJ et al (2013) Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett 13:1341–1347. doi:10.1021/Nl400258t
Zhang L, Sun L, Liu S, Huang YH, Xu KW, Ma F (2016) Effective charge separation and enhanced photocatalytic activity by the heterointerface in MoS2/reduced graphene oxide composites. RSC Adv 6:60318–60326. doi:10.1039/c6ra10923c
Pham TT, Santos CN, Joucken F, Hackens B, Raskin J-P, Sporken R (2016) The role of SiC as a diffusion barrier in the formation of graphene on Si(111). Diam Relat Mater 66:141–148. doi:10.1016/j.diamond.2016.04.011
He L, Jing L, Luan Y, Wang L, Fu H (2014) Enhanced visible activities of α-Fe2O3 by coupling N-doped graphene and mechanism insight. ACS Catal 4:990–998. doi:10.1021/cs401122e
Iamprasertkun P, Krittayavathananon A, Sawangphruk M (2016) N-doped reduced graphene oxide aerogel coated on carboxyl-modified carbon fiber paper for high-performance ionic-liquid supercapacitors. Carbon 102:455–461. doi:10.1016/j.carbon.2015.12.092
Wang C, Kang J, Sun H, Ang HM, Tade MO, Wang S (2016) One-pot synthesis of N-doped graphene for metal-free advanced oxidation processes. Carbon 102:279–287. doi:10.1016/j.carbon.2016.02.048
Asedegbega-Nieto E, Perez-Cadenas M, Morales MV et al (2014) High nitrogen doped graphenes and their applicability as basic catalysts. Diam Relat Mater 44:26–32. doi:10.1016/j.diamond.2014.01.019
Huang J, Chen F, Zhang Q et al (2015) 3D silver nanoparticles decorated zinc oxide/silicon heterostructured nanomace arrays as high-performance surface-enhanced raman scattering substrates. ACS Appl Mater Interfaces 7:5725–5735. doi:10.1021/am507857x
Wang K, Xi D, Zhou C et al (2015) CoSe2 necklace-like nanowires supported by carbon fiber paper: a 3D integrated electrode for the hydrogen evolution reaction. J Mater Chem A 3:9415–9420. doi:10.1039/c5ta01143d
Conway BE, Tilak BV (2002) Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim Acta 47:3571–3594. doi:10.1016/S0013-4686(02)00329-8
Li Y, Wang H, Xie L, Liang Y, Hong G, Dai H (2011) MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. JACS 133:7296–7299. doi:10.1021/ja201269b
Chen X, Liu G, Zheng W et al (2016) Vertical 2D MoO2/MoSe2 core-shell nanosheet arrays as high-performance electrocatalysts for hydrogen evolution reaction. Adv Funct Mater 26:8537–8544. doi:10.1002/adfm.201603674
Wang K, Ye Z, Liu C et al (2016) Morphology-controllable synthesis of cobalt telluride branched nanostructures on carbon fiber paper as electrocatalysts for hydrogen evolution reaction. ACS Appl Mater Interfaces 8:2910–2916. doi:10.1021/acsami.5b10835
Zhou H, Yu F, Huang Y et al (2016) Efficient hydrogen evolution by ternary molybdenum sulfoselenide particles on self-standing porous nickel diselenide foam. Nat Commun 7:12765. doi:10.1038/ncomms12765
Acknowledgements
This work was jointly supported by National Natural Science Foundation of China (Grant Nos. 51471130, 51771144, 51501012, 51601142), Natural Science Foundation of Shaanxi Province (No.2017JZ015),the fund of the State Key Laboratory of Solidification Processing in NWPU (SKLSP201708), and Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, L., Sun, L., Huang, Y. et al. Hydrothermal synthesis of N-doped RGO/MoSe2 composites and enhanced electro-catalytic hydrogen evolution. J Mater Sci 52, 13561–13571 (2017). https://doi.org/10.1007/s10853-017-1417-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1417-7