Abstract
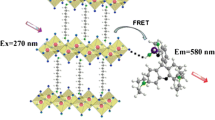
This work reports the confluence of static and dynamic fluorescence quenching of rhodamine B (RhB) by chemically exfoliated molybdenum disulfide (MoS2) nanosheets. Both steady state and time-resolved fluorescence quenching measurements were carried out to elucidate the process of energy transfer from RhB to MoS2. The interactive forces investigated through evaluation of thermodynamic parameters from temperature dependent fluorescence measurements are found to be hydrophobic in nature. The negative value of Gibbs free energy (∆G) indicates spontaneity of the adsorption process of RhB–MoS2 system. The binding affinity of RhB–MoS2 system is also investigated using UV/Vis spectrophotometer.







Similar content being viewed by others
References
Wang QH, Kourosh KZ, Kis A, Coleman JN, Strano MS (2012) Electronics and optoelectronics of two dimensional transition metal dichalchogenides. Nat Nanotechnol 7:699–712
Chhowalla M, Shin HS, Eda G, Li LJ, Loh KP, Zhang H (2013) The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat Chem 5:263–275
Rao CNR, Ramakrishna Matte HSS, Maitra U (2013) Graphene analogues of inorganic layered materials. Angew Chem Int Ed 52:13162–13185
Stengl V, Henych J, Slusna M, Ecorchard P (2014) Ultrasound exfoliation of inorganic analogues of graphene. Nanoscale Res Lett 9:1–14
Shakya J, Patel AS, Singh F, Mohanty T (2016) Composition dependent fermi level shifting of Au decorated MoS2 nanosheets. Appl Phys Lett 108:013103
Huang W, Da H, Liang G (2013) Thermoelectric performance of MX2 (M = Mo, W; X = S, Se) monolayers. J Appl Phys 113:104304
Eda G, Yamaguchi H, Voiry D, Fujita T, Chen M, Chhowalla M (2011) Photoluminescence from chemically exfoliated MoS2. Nano Lett 11:5111–5116
Mak KF, Lee C, Hone J, Shan J, Heinz TF (2010) Atomically thin MoS2: a new direct gap semiconductor. Phys Rev Lett 105:136805
Radisavljevic B, Radenovic A, Brivio J, Giacometti V, Kis A (2011) Single layer MoS2 transistors. Nat Technol 6:147–150
Butler SZ, Hollen SM, Cao L et al (2013) Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano 7(4):2898–2926
Huang Y, Guo J, Kang Y, Ai Y, Li CM (2015) Two dimensional atomically thin MoS2 nanosheets and their sensing application. Nanoscale 7:19358–19376
Stephenson T, Li Z, Olsen B, Mitlin D (2014) Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ Sci 7:209–231
Zhu CF, Zeng ZY, Li H, Li F, Fan CH, Zhang H (2013) Single layer MoS2 based nanoprobes for homogeneous detection of biomolecules. J Am Chem Soc 135:5998–6001
Li F, Huang Y, Yang Q, Zhong ZT, Li D, Wang LH, Song SP, Fan CH (2010) A graphene-enhanced molecular beacon for homogeneous DNA detection. Nanoscale 2:1021–1026
Lu CH, Li J, Liu JJ, Yang HH, Chen X, Chen GN (2010) Increasing the sensitivity and single base mismatch selectivity of the molecular beacon using graphene oxide as the nano quencher. Chem Eur J 16:4889–4894
Deokar G, Vignaud D, Arenal R, Louette P, Colomer JF (2016) Synthesis and characterization of MoS2 nanosheets. Nanotechnology 27:075604
Imanishi N, Toyoda M, Takeda Y, Yamamoto O (1992) Study on lithium intercalation into MoS2. Solid State Ion 58:333–338
Nguyen EP, Carey BJ, Daeneke T, Ou JZ, Latham K, Zhuiykov S, Zadeh KK (2015) Investigation of two solvent grinding—assisted liquid phase exfoliation of layered MoS2. Chem Mater 27:53–59
Chen W, Zhao J, Zhang J et al (2015) Oxygen-assisted chemical vapor deposition growth of large single crystal high quality monolayer MoS2. J Am Chem Soc 137(50):15632–15635
Liu Y, Liu CY, Liu Y (2011) Investigation on fluorescence quenching of dyes by graphite oxide and graphene. Appl Surf Sci 257:5513–5518
Dulkeith E, Morteani AC, Niedereichholz KTA, Feldmann J (2015) Fluorescence quenching of dye molecules near gold nanoparticles : radiative and non-radiative effects. Phys Rev Lett 89(20):203002
Lakowicz J R (ed) (2013) Principles of fluorescence spectroscopy. Springer, New York, p 243
Deng H, Yang X, Gao Z (2015) MoS2 nanosheets as an effective fluorescence quencher for DNA methyltransferase activity detection. Analyst 104(9):3210–3215
Bright FV, Munson CA (2003) Time resolved fluorescence spectroscopy for illuminating complex systems. Anal Chim Acta 500:71–104
Bakkialakshmi Selvarani P, Chenthamarai S (2013) Fluorescence quenching of rhodamine B base by two amines. Spectrochim Acta Part A 105:557–562
Ahmad A, Kurkina T, Kern K, Balasubramanian K (2009) Applications of the static quenching of rhodamine B by carbon nanotubes. Chem Phys Chem 10:2251–2255
Behabtu N, Lomeda JR, Green MJ et al (2010) Spontaneous high concentration dispersions and liquid crystals of graphene. Nat Nanotechnol 5(6):406–411
Coleman JN (2013) Liquid exfoliation of defect-free graphene. Acc Chem Res 46:14–22
Dong L, Lin S, Yang L, Zhang J, Yang C, Yang D, Lu H (2014) Spontaneous exfoliation and tailoring of MoS2 in mixed solvents. Chem Commun 50:15936–15939
Li H, Zhang Q, Yap CCR, Tay BK, Edwin THT, Olivier A, Baillargea D (2012) From bulk to monolayer MoS2: evalution of raman scattering. Adv Funct Mater 22:1385–1390
Shi Y, Huang JK, Jin L, Hsu YT, Yu SF, Li LJ, Yang HY (2013) Selective decoration of Au nanoparticles on monolayer MoS2 single crystal. Sci Rep 3:1839
Bozkurt E, Acar M, Onganer Y, Meral K (2014) Rhodamine 101-graphene oxide composites in aqueous solution: the fluorescence quenching process of rhodamine 101. Phys Chem Chem Phys 16:18276–18281
Benesi HA, Hildebrand JH (1949) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71:2703–2707
Jhonsi MA, Nithya C, Kathiravan A (2014) Probing electron transfer dynamics of pyranine with reduced graphene oxide. Phys Chem Chem Phys 16:20878–20886
Huang J, Ye L, Gao X, Li H, Xu J, Li Z (2015) Molybdenum disulfide based amplified fluorescence DNA detection using hybridization chain reaction. J Mater Chem B 3:2395–2401
Zhang XF, Li F (2012) Interaction of graphene with excited and ground state rhodamine revealed by steady state and time resolved fluorescence. J Photochem Photobiol, A 246:8–15
Rehman DS, Deb S, Ghosh SK (2015) Relativity of electron and energy transfer contributions in nanoparticle induced fluorescence quenching. J Phys Chem C 119:27145–27155
Sen T, Patra A (2008) Resonance energy transfer from rhodamine 6G to gold nanoparticles by steady-state and time-resolved spectroscopy. J Phys Chem C 112(9):3216–3222
Swathi RS, Sebastian KL (2008) Resonance energy transfer from a dye molecule to graphene. J Chem Phys 129:054703
Patel AS, Sahoo H, Mohanty T (2014) Probing the Foster resonance energy transfer between fluorescent copper nanoclusters and cobalt complex. Appl Phys Lett 105:063112
Patel AS, Mohanty T (2014) Silver nanoclusters in BSA template: a selective sensor for Hydrogen Peroxide. J Mater Sci 49:2136–2143. doi:10.1007/s10853-013-7906-4
Prins F, Goodman AJ, Tisdale WA (2014) Reduced dielectric screening and enhanced transfer in single and few layer MoS2. Nano Lett 14:6087–6091
Jennings TL, Singh MP, Strouse GF (2006) Fluorescence life time quenching near d = 1.5 nm gold nanoparticles: probing NSET validity. J Am Chem Soc 128:5462–5467
Naghibi H, Tamura A, Sturtevant JM (1995) Significant discrepancies between Van’t Hoff and calorimetric enthalpies. Proc Natl Acad Sci 92:5597–5599
Sharma P, Das MR (2013) Removal of cationic dye from aqueous solution using graphene oxide nanosheets: investigation of adsorption parameters. J Chem Eng Data 58:151–158
Zhang YZ, Dai J, Zhang XP, Yang X, Liu Y (2008) Studies of the interaction between Sudan I and bovine serum albumin by spectroscopic methods. J Mol Struct 888:152–159
Acknowledgements
The authors are thankful to AIRF, JNU, for TRFS and TEM measurements; SCNS, JNU, for fluorescence Spectroscopy; Dr. Sobhan Sen of SPS, JNU for UV/Vis absorption measurement; and IUAC, New Delhi, for Raman study. Dr. Arun Singh Patel of SCIS, JNU, is gratefully acknowledged for the discussion of results. J. S. acknowledges UGC, India, for research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shakya, J., Sahoo, H. & Mohanty, T. A study on the interaction between molybdenum disulfide and rhodamine B by spectroscopic methods. J Mater Sci 52, 3831–3840 (2017). https://doi.org/10.1007/s10853-016-0640-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0640-y