Abstract
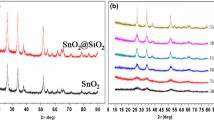
Improvement of long-term stability of electrode materials in Li-ion batteries requires a detailed understanding of influence of synthesis parameters on surface chemistry and on properties. Therefore, bare SnO2 and core/shell nanoparticles with SnO2 core and a hydrocarbon shell are synthesized in an Ar/20% O2 microwave plasma, deposited as porous nanoparticle films in situ on heated Ni-substrates, and finally assembled as anodes in Swagelok cells. In a comprehensive study, we investigate structure, particle size, chemistry, morphology, and water content of the nanoparticles using X-ray diffraction, transmission electron microscopy, specific surface area analysis, and coulometric water titration. The thicknesses of the nanoparticle films and their surface chemistry are investigated by scanning electron microscopy and X-ray photoelectron spectroscopy. SnO2 nanoparticles are crystalline, with a tetragonal cassiterite structure. Primary particle sizes around 3 nm are reached for the bare SnO2 particles, 5–8 nm for the cores of the core/shell nanoparticles. A minimum microwave power of 900 W is necessary to synthesize SnO2 nanoparticles without precursor residuals as pristine SnO2 particles for the subsequent coating step. In the coating step increasing hydrocarbon content can be correlated with increasing carbon-precursor feeding rate. Water uptake, stemming either from the process, or due to atmospheric contamination, can successfully be reduced by a thermal treatment. The still remaining water is a function of specific surface area. Finally, bare SnO2 versus core/shell nanoparticles are compared regarding the influence of the shell on the electrochemical properties. The principal improved functionality of the developed anodes in Swagelok cells is demonstrated.








Similar content being viewed by others
References
Li N, Martin CR, Scrosati B (2001) J Power Sources 97–98:240
Wang Y, Lee JY, Chen BH (2004) J Electrochem Soc 151(4):A563. doi:10.1149/1.1647571
Wang Y, Lee JY (2005) J Power Sources 144(1):220
Zhao Y, Zhou Q, Liu L, Xu J, Yan M, Jiang Z (2006) Electrochim Acta 51(13):2639
Liang Y, Fan J, Xia X, Jia Z (2007) Mater Lett 61(22):4370. doi:10.1016/j.matlet.2007.02.008
Aifantis KE, Brutti S, Hackney SA, Sarakonsri T, Scrosati B (2010) Electrochim Acta 55(18):5071. doi:10.1016/j.electacta.2010.03.083
Gao M, Chen X, Pan H, Xiang L, Wu F, Liu Y (2010) Electrochim Acta 55(28):9067. doi:10.1016/j.electacta.2010.08.033
Ochs R, Szabó DV, Schlabach S, Becker S, Indris S (2011) Phys Status Solidi A 208(2):471. doi:10.1002/pssa.201026652
Ohzuku T, Iwakoshi Y, Sawai K (1993) J Electrochem Soc 140(9):2490. doi:10.1149/1.2220849
Bruce PG, Scrosati B, Tarascon JM (2008) Angew Chem Int Ed 47(16):2930
Centi G (2009) Europ J Inorg Chem 26:3851
Chen Y-C, Chen J-M, Huang Y-H, Lee Y-R, Shih HC (2007) Surf Coat Technol 202(4–7):1313
Yao J, Shen X, Wang B, Liu H, Wang G (2009) Electrochem Commun 11(10):1849
Du Z, Yin X, Zhang M, Hao Q, Wang Y, Wang T (2010) Mater Lett 64(19):2076
Qiao H, Zheng Z, Zhang L, Xiao L (2008) J Mater Sci 43(8):2778. doi:10.1007/s10853-008-2510-8
Vollath D, Szabó DV (2006) J Nanopart Res 8(3–4):417. doi:10.1007/s11051-005-9014-0
Schumacher B, Ochs R, Tröße H, Schlabach S, Bruns M, Szabó DV, Haußelt J (2007) Plasma Process Polym 4(S1):S865. doi:10.1002/ppap.200732101
Dato A, Radmilovic V, Lee Z, Phillips J, Frenklach M (2008) Nano Lett 8(7):2012. doi:10.1021/nl8011566
Marcinek M, Hardwick LJ, Zukowska GZ, Kostecki R (2010) Carbon 48(5):1552
Moreno-Couranjou M, Monthioux M, Gonzalez-Aguilar J, Fulcheri L (2009) Carbon 47(10):2310
Peter S, Günther M, Hauschild D, Grambole D, Richter F (2010) Vacuum 85(4):510. doi:10.1016/j.vacuum.2010.10.006
Koprinarov N, Konstantinova M (2011) J Mater Sci 46(5):1494. doi:10.1007/s10853-010-4951-0
Park M, Kang Y, Kim J, Wang G, Dou S, Liu H (2008) Carbon 46(1):35. doi:10.1016/j.carbon.2007.10.032
Parry KL, Shard AG, Short RD, White RG, Whittle JD, Wright A (2006) Surf Interface Anal 38(11):1497. doi:10.1002/sia.2400
Scofield JH (1976) J Electron Spectrosc Relat Phenom 8(2):129. doi:10.1016/0368-2048(76)80015-1
Tanuma S, Powell CJ, Penn DR (1994) Surf Interface Anal 21(3):165. doi:10.1002/sia.740210302
Schlabach S, Szabó DV, Vollath D, de la Presa P, Forker M (2007) J Alloy Compd 434:590. doi:10.1016/j.jallcom.2006.08.087
Schlabach S, Szabó DV, Vollath D, Braun A, Clasen R (2004) Funct Nanomater Optoelectronics Other Appl 99–100:191
Vollath D, Szabó DV (2002) In: Choy K-L (ed) Innovative processing of films and nanocrystalline powders. Imperial College Press, London, p 219
Brossmann U, Sagmeister M, Polt P, Kothleitner G, Letofsky-Papst I, Szabó DV, Würschum R (2007) Phys Status Solidi RRL 1(3):107. doi:10.1002/pssr.200701019
Szabó DV, Schlabach S, Ochs R (2007) In: Gemming T, Hartmann U, Mestres P, Walther P (eds) Microscopy Conference 2007, Saarbrücken, Germany, 2007. Microscopy and Analysis. Cambridge University Press, vol 13, Supplement 3 p 430
Fuchs M, Breitenstein D, Fartmann M, Grehl T, Kayser S, Koester R, Ochs R, Schlabach S, Szabó DV, Bruns M (2010) Surf Interface Anal 42(6–7):1131
Vollath D, Szabó DV, Schlabach S (2004) J Nanopart Res 6(2–3):181
Lamparth I, Szabó DV, Vollath D (2002) Macromol Symp 181:107
Szabó DV, Lamparth I, Vollath D (2002) Macromol Symp 181:393
Powell RA (1979) Appl Surf Sci 2:397
Hoflund GB, Corallo GR (1992) Phys Rev B 46(11):7110
JCPDS 41-1445. The International Centre for Diffraction Data, Newton Square, PA, USA
Yu KN, Xiong YH, Liu YL, Xiong CS (1997) Phys Rev B 55(4):2666
Lock EH, Petrovykh DY, Mack P, Carney T, White RG, Walton SG, Fernsler RF (2010) Langmuir 26(11):8857. doi:10.1021/la9046337
Stevens JS, Schroeder SLM (2009) Surf Interface Anal 41(6):453. doi:10.1002/sia.3047
Stankovich S, Piner RD, Chen X, Wu N, Nguyen ST, Ruoff RS (2006) J Mater Chem 16(2):155
Li F, Song J, Yang H, Gan S, Zhang Q, Han D, Ivaska A, Niu L (2009) Nanotechnology 20(45):455602
Moulder JF, Stickele WF, Sobol PE, Bomben KD (1992) Handbook of X-ray photolelectron spectroscopy. Perkin Elmer Corp., Eden Prairie
Trouillet V, Tröße H, Bruns M, Nold E, White RG (2007) J Vac Sci Technol A 25(4):927. doi:10.1116/1.2731342
Ma Y, Castro RHR, Zhou W, Navrotsky A (2011) J Mater Res 26(7):848. doi:10.1557/jmr.2010.97
Mersmann A, Kind M, Stichlmair J (2005) Thermische Verfahrenstechnik, 2nd edn. Springer, Berlin
Liu HP, Long DH, Liu XJ, Qiao WM, Zhan L, Ling LC (2009) Electrochim Acta 54(24):5782. doi:10.1016/j.electacta.2009.05.030
Hassoun J, Derrien G, Panero S, Scrosati B (2008) Adv Mater 20(16):3169. doi:10.1002/adma.200702928
Mohamedi M, Lee S-J, Takahashi D, Nishizawa M, Itoh T, Uchida I (2001) Electrochim Acta 46(8):1161. doi:10.1016/s0013-4686(00)00702-7
Demir-Cakan R, Hu Y-S, Antonietti M, Maier J, Titirici M-M (2008) Chem Mater 20(4):1227. doi:10.1021/cm7031288
Deng D, Lee JY (2008) Chem Mater 20(5):1841. doi:10.1021/cm7030575
Sun X, Liu J, Li Y (2006) Chem Mater 18(15):3486. doi:10.1021/cm052648m
Kim C, Noh M, Choi M, Cho J, Park B (2005) Chem Mater 17(12):3297. doi:10.1021/cm048003o
Acknowledgements
The authors acknowledge financial support by the German Ministry for Education and Research, project “Battery Competence Consortium South—Electrochemistry for Electromobility” under contract number 03KP801.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szabó, D.V., Kilibarda, G., Schlabach, S. et al. Structural and chemical characterization of SnO2-based nanoparticles as electrode material in Li-ion batteries. J Mater Sci 47, 4383–4391 (2012). https://doi.org/10.1007/s10853-012-6292-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-012-6292-7