Abstract
Understanding the fundamental aspects of transport through biomaterials is a necessity for a vast range of bio-related studies. Biofilm formation on the surface of adsorptive media such as granular activated carbon (GAC) has been extensively used to remove organic materials, nitrogen species, heavy metals, and other contaminants in wastewater treatment. In this study, a multilayer mass transfer system consisting of the reactor’s bulk fluid, diffusion layer, biofilm, and GAC is modeled. In order to consider the equilibrium at the interface of biofilm and activated carbon, Freundlich adsorption method is applied. The interfacial interactions are taken into account in the biodegradation process. The results of model prediction are compared with the available experimental data and show a very good agreement. The effect of biofilm formation on the reactor porosity is considered through a porous media approach. Furthermore, the influence of variation in particle diameters on the removal efficiency is studied. It can be seen that porosity alteration as a result of biofilm formation within the carbon bed has a noticeable effect on the removal efficiency.
Similar content being viewed by others
Introduction
Biofilm is a dominant form of existence for bacteria in most natural and synthetic environments. Depending on the application area, they can be useful or harmful. They are helpful in bioremediation, microbial enhanced oil recovery, and metal extraction. On the other hand, biofilms are damaging for water pipes, heat exchangers, submarines, and body organs [1, 2]. Granular activated carbon (GAC) has been used as an adsorbent medium to extract a wide range of materials from liquid solutions or gaseous mixtures. They are highly porous and their large interior surfaces make them favorite adsorptive filters. Biofilm formation on the surface of GAC substantially increases its treatment efficiency in different applications [3–8]. Understanding the details of biofilm mechanisms and characteristics will lead to a more precise knowledge of contaminants fate in many natural and synthetic environments.
Scott et al. [9] performed an experimental study on decontamination of streams containing heavy metals and organic chemicals by GAC covered with biofilm. They concluded that carbon by itself is not an efficient adsorbent for heavy metals but with the aid of biofilm and its EPS, rate and quantity of metal extraction substantially increases. Liang et al. [3] studied the adsorption and biodegradation of chemical compounds on biological activated carbon (BAC) utilizing a continuous column with different media such as GAC, seeded glass bead, and seeded GAC. They concluded that adsorption is the prevailing mechanism for removal of the contaminating compound and biodegradation is the main key to eradicate ozonation intermediates. Ravindran et al. [10] studied mathematical models incorporating adsorption and mass transfer, and developed biofilm degradation for performance prediction of bioactive carbon fixed-bed and fluidized-bed adsorbers in wastewater treatment. Sensitivity analysis was performed to evaluate the effects of adsorption equilibrium, mass transfer, biokinetic and biofilm parameters.
Yang and Al-Duri [11] studied adsorption equilibrium and kinetics of three reactive dyes from their single-component aqueous solutions onto activated carbon in a batch reactor. They found out a kinetic model which generated the best agreement with the three adsorption systems’ experimental data. Chudyk and Snoeyink [12] studied bioregeneration of GAC under conditions of typical water treatment plant operations. Kim et al. [13] developed a mathematical model that describes the adsorption and biodegradation phenomena in fluidized-bed absorbers. They observed that the model is satisfactory for an ideal biodegradable compound and wastewaters consisting of a large range of substrates. Liang et al. [14] focused on differentiating the adsorption and biodegradation quantities of a biologically activated carbon column and on evaluating the effect of empty bed contact time on the performance of these two mechanisms simultaneously for the removal of dissolved organic matter from water. They observed that the empty bed contact time could influence the performance of adsorption and biodegradation. Chang and Rittmann [7] studied the effect of attaching surface on biofilm shear loss with two different types of granular activated carbons. They observed that the shear loss is a function of surface coverage by biofilm and that it notably changes when the carbon is completely covered.
The biofilm interaction with metals has been studied in a wide range of applications [9, 15–19]. Malik [15] reported the successful and economically efficient removal of heavy metals by bacterial biofilms. Multispecies biofilms have shown more efficacies for different pH ranges and high metal concentrations. The extracellular polymeric substance (EPS) of the biofilm entraps the dissolved metals. Puranik et al. [16] investigated the biosorption of lead and zinc and interpreted the experimental data with a mathematical model based on the equilibrium of metal sorption and mass transfer kinetics. Lameiras et al. [17] studied the biosorption of Cr(VI) in different configuration of GAC and zeolite columns. The absorber media were covered with Arthrobacter viscous biofilm. They investigated the interactions among metal ions and functional group in the biomass utilizing Fourier transform infrared spectroscopy (FTIR).
The objective of this work is to study the chemical substrate removal through a multilayer mass transfer system in a reactor consisting of GAC covered with biofilm. The presented model takes into account the fundamental mechanisms of transport in bulk fluid, diffusion layer, biofilm, and GAC. Combination of all these mechanisms along with accounting for the interfacial phenomena provides us with a model which is able to predict the efficiency of biosorption for water decontamination. The effect of biofilm formation on the porosity of the carbon bed is considered. Biofilm occupies the available empty spaces in the GAC bed which influences the contaminant removal efficiency as our results show. To the authors’ best knowledge, the porosity changes in the GAC bed has not been considered in any of the previous studies in this field.
Materials and methods
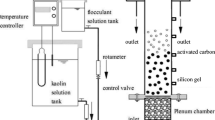
The simple schematic of multilayer mass transfer system including bulk fluid, diffusion layer, biofilm, and carbon can be seen in Fig. 1.
Bulk fluid
It is assumed that the biosorption occurs in a completely mixed biofilm reactor. A mass balance for the reactor provides us with the following equations for phenol and bacteria concentrations in the bulk fluid [5].
where C bulk is phenol concentration in the bulk fluid (Mph/L3), Q is the volumetric flow rate of the feed solution (L3/T), V reactor volume (L3), ɛ porosity of the reactor, Y yield of biomass (Mx/Mph), k maximum rate of substrate utilization (Mph/(MxT)), W c weight of carbon in the reactor (Mc), h m diffusion layer mass transfer coefficient (L/T), R c carbon particle diameter (L), ρc density of the carbon particle (Mc/L3), L f biofilm thickness (L), K s half-velocity concentration (Mph/L3), X bulk bacterial concentration in the bulk fluid (Mx/L3) and X f bacterial concentration in the biofilm (Mx/L3).
Biofilm phase
The biofilm model is based on the substrate diffusion and single Monod reaction [20] as can be seen below:
where C f is the substrate concentration in biofilm (Mph/L3), D f substrate diffusion coefficient in the biofilm (L2/T), and r f is the biofilm coordinate (L).
Biofilm boundary conditions
Substrate concentration at the surface of GAC is obtained from the Freundlich equilibrium as follows:
where m c is carbon adsorption capacity for phenol at the interface of biofilm and GAC (Mph/Mc), and K c is the Freundlich isotherm coefficient \( \left( {{\text{M}}_{\text{ph}}^{1 - 1/n} {\text{L}}^{ - 3/n} /{\text{M}}_{\text{c}} } \right) .\)
At the interface of biofilm and diffusion layer the mass flux continuity requires:
where h m is the mass transfer coefficient in the diffusion layer taken form Williamson et al. [21] work based on the following correlation:
where v s is the superficial velocity in the reactor, ɛ porosity of the reactor, Re Reynolds number \( \left( {{\frac{{\rho (d_{\text{c}} + L_{\text{f}} )v_{\text{s}} }}{\mu }}} \right) \); d c is the carbon diameter; Sc is Schmidt number \( \left( {{\frac{\mu }{\rho D}}} \right) \), and D is the diffusivity of water. It should be noted that due to variances in mass transfer relationships, other correlations are also considered in this work.
Biofilm growth
As the biofilm starts consuming the substrate, microorganisms continue their biosynthesis and respiration. Biofilm thickness variation versus time is a net effect of interactions among microbial growth, microbial decay, and shear stress as can be seen in the following equation:
where b is the biofilm decay coefficient (1/T) and b s is biofilm shear loss coefficient (1/T).
Solid phase
The diffusion equation is used to account for the transport of substrate through the carbon particles assuming that no reaction occurs within the sphere.
where m ph,c is the carbon adsorption capacity for phenol (Mph/Mc), D c substrate diffusion coefficient in the carbon (L2/T), and r c radial coordinate of the carbon particle (L), where M, L and T refer to mass, length, and time units, respectively.
The symmetry of the concentration profile within the sphere and Freundlich equilibrium at the carbon biofilm interface lead to the following boundary conditions:
where K c is the Freundlich isotherm coefficient \( \left( {{\text{M}}_{\text{ph}}^{1 - 1/n} {\text{L}}^{ - 3/n} /{\text{M}}_{\text{c}} } \right) .\) Conservation of mass over GAC requires that the substrate mass entering the carbon particle has to be equal to the increase of mass storage inside the carbon particle. This results:
Equations 1–11 take into account the fundamental substrate transport aspects through a multilayer system. This model considers the reaction and attachment in the bulk fluid, transport through the diffusion layer, diffusion and reaction in the biofilm, and diffusion within the spherical carbon particle. As mentioned before the reactor is assumed to be completely mixed and all the carbon particles are considered to have the same diameter.
Biofilm affected porosity
In order to study the effect of biofilm formation on the porosity of GAC bed, a cubic unit cell is chosen to represent the porous media and it is assumed that biofilm forms homogenously on the spherical surfaces.
The following relationship [1, 22] gives the biofilm affected porosity of the porous media represented by a cubic unit cell.
where α is the arrangement factor of the unit cell, d c the carbon diameter, m the number of tangential contacts for each sphere with its neighbors within the unit cell and V sb is the volume occupied by carbon spheres and the biofilm formed over their surfaces. Figure 2a shows the unit cell that is utilized to represent the porous media network. It is assumed that biofilm homogenously covers the surface of spherical particles as can be seen in Fig. 2b.
Since the mass transfer coefficient is a function of the reactor porosity based on Eq. 6 as well as other mass transfer correlations (given in Eqs. 13 and 14), it changes with the biofilm formation inside the bed as well. We have utilized two other mass transfer correlations to show the effect of biofilm formation on removal efficiency. The following correlations were obtained from the works of Frantz [23] and Upadhyay and Tripathi [24], respectively.
Frantz (1962)
Upadhyay and Tripathi (1975)
The removal efficiency for the substrate is calculated from
Results and discussions
Phenol concentration in the bulk fluid obtained from the model calculations is compared with the experimental data given in Chang and Rittmann’s [6] work, utilizing the physical parameters used in their model. As seen in Fig. 3, model predictions are in a very good agreement with the experimental data except an overestimation for the peak of the curve that occurs around 45 h after phenol injection into the feed solution. Figure 3 has the typical characteristics of an adsorptive carbon breakthrough curve, which displays almost negligible biofilm consumption before the peak. Biofilm growth catches up shortly after the maximum point and a substantial substrate concentration decrease occurs in the bulk fluid. Eventually, the substrate concentration reaches an almost constant level which can be considered as the final response of the treatment system.
Comparison of the model-based predicted data with experimental results of Chang and Rittmann [5] for phenol normalized concentration in the bulk fluid
The GAC is able to absorb the substrate from the biofilm and desorb into it. Figure 4 shows the profile of carbon adsorption capacity versus time at the center and the external surface of the particle. It can be seen that the adsorption turns to desorption sometime around the first day and finally carbon becomes saturated and m ph,c at the surface and center of the sphere become identical. In Fig. 5a and b, the spatial profile of adsorption capacity is shown after 1 and 4 days.
The formation of biofilm over the carbon particles leads to a reduction of the available empty spaces in the reactor which alters its porosity based on Eq. 12. As can be seen in Fig. 6, the mass transfer coefficient and removal efficiency change when accounting for the effect of biofilm formation on the reactor porosity. The diffusion layer mass transfer coefficient becomes smaller based on Eq. 6 (Williamson et al. correlation) and its magnitude will be affected by decreasing porosity due to the growth of biofilm thickness. Therefore, this causes an accelerated decreasing trend for the biofilm mass transfer coefficient, h mb as seen in Fig. 6a. The removal efficiency of the system will be influenced by changes in mass transfer coefficient as shown in Fig. 6b.
The removal efficiency and mass transfer coefficient while considering and neglecting the effect of porosity alteration due to the biofilm formation is illustrated in Figs. 7 and 8 based on the other mass transfer correlation equations given in Eqs. 13 and 14. It can be seen that the porosity variation has a different influence on the mass transfer coefficient and removal efficiency of the system for these correlations. Since this method is used to a large extent in the water treatment, even a small miscalculation in the phenol content or other contaminants in the filtered water results in a substantial difference. Therefore, proper accounting of the porosity variation during the treatment process and its subsequent influence on the mass transfer through different layers is necessary to capture a more realistic picture of the described physical event.
Figure 9a and b displays the removal efficiency and mass transfer coefficient profiles for three different particle diameters based on Williamson et al. and Upadhyay and Tripathi correlations. These figures show the effect of carbon particle size on mass transfer coefficient ratio and removal efficiency of the system. The minimum change in the mass transfer coefficient occurs for the smallest carbon particles regardless of whether Williamson et al. or Upadhyay and Tripathi correlations are utilized as seen in Fig. 9b. Removal efficiency, which is affected by the biofilm growth is shown in Fig. 9a. As can be seen in Fig. 9a, the highest removal efficiency corresponds to the smallest size particles. This falls in line with the physical expectations. As can be seen in Eq. 1, the attachment term becomes larger when the particle diameter becomes smaller. This is due to enhancement of available contact surface for the case of small particles in the reactor. The sharp increase in the removal efficiency after approximately the first 100 h is related to the biofilm growth domination. Finally, biofilm thickness growth for different GAC sizes can be seen in Fig. 10, which also shows the least growth for the smallest size particles. This will in turn result in smaller porosity variations for the smaller size particles.
Conclusions
A comprehensive model was utilized to study the contaminant removal within a bed of GCA in a mixed reactor. The presented model accounted for the phenol removal from the contaminated water. The fundamental aspects of mass transport through a multilayer system including the bulk fluid, diffusion layer, biofilm, and the carbon particle along with the interfacial interactions among the layers is taken into account. The model predictions displayed a very good agreement when compared with the available experimental data in literature. The effect of reactor porosity alteration due to the formation of biofilm on the carbon particles was accounted for by utilizing a porous media approach. Previous studies in this field [3–8] took the reactor porosity constant during the biosorption process. The mass transfer coefficient in the diffusion layer is influenced by the porosity variation in the bed and the removal efficiency becomes affected as a result of less available empty space in the reactor. Since this method of treatment is utilized extensively for drinking water, a small change in the concentration of the chemical compound in the solution makes a noticeable difference. The effect of porosity variation on the mass transfer coefficient is contradictory when utilizing different correlations from the literature. There is a need for clarification of the available correlations so as to obtain more precise modeling prediction in biosorption.
Finally, the effect of carbon particle diameter on the removal efficiency was also studied. It was established that reducing the particle size increases the removal efficiency of the reactor.
References
Shafahi M, Vafai K (2009) Int J Heat Mass Transf 52:574
Shafahi M, Vafai K (2010) Int J Heat Mass Transf 53:2943
Liang CH, Chiang PC, Chang EE (2003) Ozone Sci Eng 25:351
Liang CH, Chiang PC, Chang EE (2007) Water Res 41:3241
Chang HT, Rittmann BE (1987) Environ Sci Technol 21:273
Chang HT, Rittmann BE (1987) Environ Sci Technol 21:280
Chang HT, Rittmann BE (1988) Water Pollut Control Fed 60:362
Badriyha BN, Ravindrann V, Den W, Pirbazari M (2003) Water Res 37:4051
Scott JA, Karanjkar AM, Rowe DL (1995) Miner Eng 8:221
Ravindran V, Badriyha BN, Pirbazari M, Kim S-H (1996) Appl Math Comput 76:99
Yang X, Al-Duri B (2005) J Colloids Interface Sci 287:25
Chudyk WA, Snoeynik VL (1984) Environ Sci Technol 18:1
Kim S-H, Pirbazari M (1989) J Environ Eng 115:1235
Liang CH, Chiang PC, Chang EE (2004) J Chin Inst Chem Eng 35:203
Malik A (2004) Environ Int 30:261
Puranik PR, Modak JM, Paknikar KM (1999) Hydrometallurgy 52:189
Lameiras S, Quintelas C, Tavares T (2008) Bioresour Technol 99:801
Ferris FG, Schultze S, Witten TC, Fyfe WS, Beveridge TJ (1989) Appl Environ Microbiol 55:1249
Teitzel GM, Parsek MR (2003) Appl Environ Microbiol 69:2313
Rittmann BE, McCarty PL (1981) J Environ Eng Div 107:831
Williamson JE, Bazaire KE, Geankoplis CJ (1963) J Ind Eng Chem Fundam 2:126
Taylor SW, Milly PCD, Jaffe P (1990) Water Resour Res 26:2161
Frantz J (1962) Chem Eng 69:161
Upadhyay SN, Tripathi G (1975) J Chem Eng Data 20:20
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Shafahi, M., Vafai, K. Interfacial interactions of biomaterials in water decontamination applications. J Mater Sci 46, 6277–6284 (2011). https://doi.org/10.1007/s10853-011-5417-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-011-5417-8