Abstract
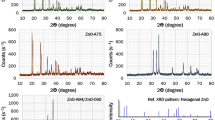
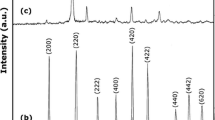
The effect that the phase of the starting nanoseed titania (TiO2), the pH of the solvent solution, and the processing methodology employed have on the properties of the resultant TiO2 nanomaterials were explored. This led to the development of a new process to produce large-scale, phase pure, thin nanowires of TiO2 at high pH and nanosquares at low pH. Anatase, rutile, and Degussa P25TM TiO2 nanoparticle starting materials (or nanoseeds) were processed in strongly basic (10 M KOH) and strongly acidic (conc. HX, where X = Cl, Br, I) solutions using solvothermal (SOLVO) and solution precipitation (SPPT) methodologies. Under basic SOLVO conditions, the nanoseeds were converted to H2Ti2O5·H2O nanowires. The SPPT basic conditions also produced the same phased nanowires for the rutile and anatase nanoseeds, while the Degussa nanomaterial yielded mixed phased [anatase:rutile (9:1)] nanowires. The SPPT method was found to produce substantially thinner nanowires in comparison to the SOLVO route, with comparable surface areas but the strong basic media led to etching of the glassware yielding HK3Ti4O4(SiO4)3·4H2O nanorods. Hybridization of these two processing routes led to the use of NalgeneTM bottle as the reaction flask termed the hybrid (HYBR) route, yielding even thinner H2Ti2O5·H2O nanowires on a large-scale. Switching to a concentrated halide acid (HX, where X = Cl, Br, I) system, SOLVO, SPPT, and HYBR routes were investigated. The resultant TEM images revealed that the rutile starting material yielded short rods, whereas the anatase seeds formed square or faceted materials.












Similar content being viewed by others
References
Kislyuk VV, Dimitriev OP (2008) J Nanosci Nanotech 8:131
Zhu BL, Li KO, Zhang SM, Wu SH, Huang WP (2008) Prog Chem 20:48
van de Krol R, Liang YQ, Schoonman J (2008) J Mater Chem 18:2311
Qui JJ, Yu WD, Gao XD, Li XM, He WZ, Park SJ, Kim HK, Hwang YH (2008) J Sol-Gel Sci Tech 47:187
Masuda Y, Kato K (2008) Cryst Growth Des 8:3213
Ban T, Nakatani T, Uehara Y, Ohya Y (2008) Cryst Growth Des 8:935
Bavyin DV, Cressey BA, Walsh FC (2007) Aust J Chem 60:95
Yuan ZY, Su BL (2004) Coll Surf A—Phys Engin Asp 241:173
Wang BX, Shi Y, Xue DF (2007) J Solid State Chem 180:1028
Khan SUM, Sultana T (2003) Solar Energy Mater Solar Cells 76:211
Hakuta Y, Hayashi H, Arai K (2004) Mater Res Soc Symp Proc 789:263
Dong W, Cogbill A, Zhang T, Ghosh S, Tian ZR (2006) J Phys Chem B Lett 110:16819
Tian ZR, Voigt JA, Liu J, McKenzie B, Xu H (2003) J Am Chem Soc 125:12384
Penn RL, Banfield JF (1999) Geochimica Cosmochim Acta 63:1549
Gao Y, Elder SA (2000) Mater Lett 44:228
Chemseddine A, Mortiz T (1999) Eur J Inorg Chem 2:235
Sugimoto T, Zhou X, Muramatsu A (2003) J Colloid Interface Sci 259:43
Finnegan MP, Zhang H, Banfield JF (2007) J Phys Chem C 111:1962
Li Y, White TJ, Lim SH (2004) J Solid State Chem 177:1372
Sugimoto T, Zhou X, Muramatsu A (2003) J Colloid Interface Sci 259:53
Zaban A, Aruna ST, Tirosh S, Gregg BA, Mastai Y (2000) J Phys Chem B 104:4130
Aruna ST, Tirosh S, Zaban A (2000) J Mater Chem 10:2388
Barnard AS, Curtiss LA (2005) NanoLetters 5:1261
Roy SC, Paulose M, Grimes CA (2007) Biomaterials 28:4667
Bright E, Readey DW (1987) J Am Ceram Soc 70:900
Schmuki P, Bauer S, Kleber S (2006) Electrochm Commun 8:1321
Wu G, Wang J, Thomas DF, Chen A (2008) Langmuir 24:3503
Lan Y, Gao XD, Zhu H, Zheng Z, Yan T, Wu F, Ringer SP, Song D (2005) Adv Funct Mater 15:1310
Chen XB, Mao SS (2006) J Nanosci Nanotech 6:906
Chen XB, Mao SS (2007) Chem Rev 107:2891
Tachikawa T, Fujitsuka M, Majima T (2007) J Phys Chem C 111:5259
Jwo CS, Tien DC, Teng TP, Chang H, Tsung TT, Liao CY, Lin CH (2005) Rev Adv Mater Sci 10:283
Tomovska R, Marinkovski M, Frajgar R (2007) NATO Sci Peace Security Series C-Envir Security 207
Swamy V (2008) Phys Rev B 77:195414
Sasaki T, Shimizu Y, Koshizaki N (2005) Rev Laser Engin 33:18
Theron J, Walker JA, Cloete TE (2008) Crit Rev Microbiol 34:43
Bogue RW (2004) Sensor Rev 24:253
Bruce PG, Scrosati B, M TJ (2008) Angew Chem IEEE 47:2930
Jing LQ, Qu YC, Wang BQ, Li SD, Jiang BJ, Yang LB, Fu W, Fu HG, Sun JZ (2006) Solar Energy Mater Solar Cells 90:1773
Hulteen JC, Martin CR (1997) J Mater Chem 7:1075
Ofir Y, Samanta B, Rotello VM (2008) Chem Soc Rev 37:1814
Balazs AC, Emrick T, Russell TP (2006) Science 314:1107
Vaia RA, Maguire JF (2007) Chem Mater 19:2736
Mackay ME, Tuteja A, Duxbury PM, Hwawker CJ, Van Horn B, Guan Z, Chen G, Krishnan RS (2006) Science 311:1740
Jade XRD Pattern Processing MDI, Inc., Livermore, CA (1999)
Daoud WA, Pang GKH (2006) J Phys Chem B 110:25746
Sugita M, Tsugi M, Abe M (1990) Bull Chem Soc Jpn 63:1978
Mer’kov AN, Bussen IV, Goiko EA, Kul’chitskaya EA, Men’shikov YP, Nedorezova AP (1973) Zap Vses Miner O-va 102:54
Valtchev V, Paillaud J-L, Mintova S, Kessler H (1999) Microporous Mesoporous Mater 32:287
Sandomirskii PA, Belov NV (1979) Sov Phys Crystallogr 24:686
Barnard AS, Xu H (2008) ACSNano 2:2237
Yeredla RR, Xu H (2008) Nanotechnology 19:1
Machesky ML, Wesolowski DJ, Fidley MK, Palmer DA, Rosenqvist J, Lvov SN, Fedkin M, Predota M, Vlcek L (2008) ECS Trans 11:151
Buchanan RC, Park T (1997) Materials crystal chemistry. Marcel Dekker, Inc., New York
Selloni A (2008) Nat Mater 7:613
Watanabe T, Nakajima A, Wang R, Minabe M, Koizumi S, Fujishima A, Hashimoto K (1999) Thin Solid Films 351:260
Acknowledgements
For support of this research, the authors thank the U.S. Department of Energy, Office of Basic Energy Science, Division of Material Sciences and Engineering and the Laboratory Directed Research and Development (LDRD) program at Sandia National Laboratories. Sandia is a multiprogram laboratory operated by Sandia Corporation, a Lockheed Martin Company, for the United States Department of Energy’s National Nuclear Security Administration under Contract DE-AC04-94AL85000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boyle, T.J., Lambert, T.N., Pratt, H.D. et al. Morphological and phase dependence of nanotitania materials generated under extreme pH conditions for large scale production of TiO2 nanowires (basic) and nanosquares or nanrods (acidic). J Mater Sci 45, 1744–1759 (2010). https://doi.org/10.1007/s10853-009-4148-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-009-4148-6