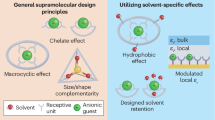
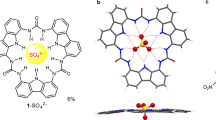
Abstract
This micro review covers recent advances in anion recognition, such as selective developments in the receptor design. Applications to which anion receptors can be applied, for example anion extraction and transport, are highlighted.




Similar content being viewed by others
References
Gale, P.A., Howe, E.N.W., Wu, X.: Anion receptor chemistry. Chem 1(3), 351–422 (2016). doi:10.1016/j.chempr.2016.08.004
Wenzel, M., Hiscock, J.R., Gale, P.A.: Anion receptor chemistry: highlights from 2010. Chem. Soc. Rev. 41, 480–520 (2012)
Gale, P.A., Busschaert, N., Haynes, C.J.E., Karagiannidis, L.E., Kirby, I.L.: Anion receptor chemistry: highlights from 2011 and 2012. Chem. Soc. Rev. 43(1), 205–241 (2014). doi:10.1039/c3cs60316d
Zwicker, V.E., Liu, X., Yuen, K.K.Y., Jolliffe, K.A.: Triazole–containing zinc(II)dipicolylamine-functionalised peptides as highly selective pyrophosphate sensors in physiological media. Supramol. Chem. 28(1–2), 192–200 (2016). doi:10.1080/10610278.2015.1122789
Zhong, D.-C., Lu, T.-B.: Molecular recognition and activation by polyaza macrocyclic compounds based on host-guest interactions. Chem. Commun. 52(68), 10322–10337 (2016). doi:10.1039/c6cc03660k
Toure, M., Charles, L., Chendo, C., Viel, S., Chuzel, O., Parrain, J.-L.: Straightforward and controlled shape access to efficient macrocyclic imidazolylboronium anion receptors. Chem. Eur. J. 22(26), 8937–8942 (2016). doi:10.1002/chem.201601174
Savastano, M., Bazzicalupi, C., Garcia, C., Gellini, C., Lopez de la Torre, M.D., Mariani, P., Pichierri, F., Bianchi, A., Melguizo, M.: Iodide and triiodide anion complexes involving anion-π interactions with a tetrazine-based receptor. Dalton Trans. 46(14), 4518–4529 (2017). doi:10.1039/c7dt00134g
Savastano, M., Bazzicalupi, C., Giorgi, C., García-Gallarín, C., López de la Torre, M.D., Pichierri, F., Bianchi, A., Melguizo, M.: Anion complexes with tetrazine-based ligands: formation of strong anion–π interactions in solution and in the solid state. Inorg. Chem. 55(16), 8013–8024 (2016). doi:10.1021/acs.inorgchem.6b01138
Ruiz-Botella, S., Vidossich, P., Ujaque, G., Peris, E.: Rim, side arms, and cavity: three sites for the recognition of anions by tetraazolium resorcinarene cavitands. Chem. Eur. J. 22(44), 15800–15806 (2016). doi:10.1002/chem.201602916
Bhat, M.P., Jung, H.-Y., Losic, D., Kurkuri, M.D.: Anion sensors as logic gates: a close encounter? Chem. Eur. J. 22(18), 6148–6178 (2016). doi:10.1002/chem.201504396
Clarke, H.J., Van Rossom, W., Horton, P.N., Light, M.E., Gale, P.A.: Anion transport and binding properties of N N′-(phenylmethylene)dibenzamide based receptors. Supramol. Chem. 28(1–2), 10–17 (2016). doi:10.1080/10610278.2015.1034126
Kuwajima, S., Kikukawa, Y., Hayashi, Y.: Small-molecule anion recognition by a shape-responsive bowl-type dodecavanadate. Chem. Asian J. 12(15), 1909–1914 (2017). doi:10.1002/asia.201700489
Vargas-Zúñiga, G.I., Sessler, J.L.: Pyrrole N–H anion complexes. Coord. Chem. Rev. 345, 281–296 (2017). doi:10.1016/j.ccr.2017.04.004
Busschaert, N., Caltagirone, C., Van Rossom, W., Gale, P.A.: Applications of supramolecular anion recognition. Chem. Rev. 115(15), 8038–8155 (2015). doi:10.1021/acs.chemrev.5b00099
Gale, P.A., Davis, J.T., Quesada, R.: Anion transport and supramolecular medicinal chemistry. Chem. Soc. Rev. 46(9), 2497–2519 (2017). doi:10.1039/c7cs00159b
Li, H., Valkenier, H., Judd, L.W., Brotherhood, P.R., Hussain, S., Cooper, J.A., Jurček, O., Sparkes, H.A., Sheppard, D.N., Davis, A.P.: Efficient, non-toxic anion transport by synthetic carriers in cells and epithelia. Nat. Chem. 8(1), 24–32 (2016). doi:10.1038/nchem.2384
Gloe, K., Stephan, H., Grotjahn, M.: Where is the anion extraction going? Chem. Eng. Technol. 26(11), 1107–1117 (2003). doi:10.1002/ceat.200306105
Gloe, K., Gloe, K., Wenzel, M., Lindoy, L.F., Li, F.: Supramolecular chemistry in solvent extraction. In: Moyer, B. A. (ed) Ion Exchange and Solvent Extraction. Ion Exchange and Solvent Extraction Series, pp. 1–48. CRC Press, Boca Raton (2013)
Ahmed, B.M., Calco, B., Mezei, G.: Tuning the structure and solubility of nanojars by peripheral ligand substitution, leading to unprecedented liquid-liquid extraction of the carbonate ion from water into aliphatic solvents. Dalton Trans. 45(20), 8327–8339 (2016). doi:10.1039/c6dt00847j
Warr, R.J., Bell, K.J., Gadzhieva, A., Cabot, R., Ellis, R.J., Chartres, J., Henderson, D.K., Lykourina, E., Wilson, A.M., Love, J.B., Tasker, P.A., Schroder, M.: A comparison of the selectivity of extraction of [PtCl6]2– by mono-, bi-, and tripodal receptors that address its outer coordination sphere. Inorg. Chem. 55(12), 6247–6260 (2016). doi:10.1021/acs.inorgchem.6b00848
Carreira-Barral, I., Mato-Iglesias, M., de Blas, A., Platas-Iglesias, C., Tasker, P.A., Esteban-Gomez, D.: Ditopic receptors containing urea groups for solvent extraction of Cu(II) salts. Dalton Trans. 46(10), 3192–3206 (2017). doi:10.1039/c7dt00093f
Fowler, C.J., Haverlock, T.J., Moyer, B.A., Shriver, J.A., Gross, D.E., Marquez, M., Sessler, J.L., Hossain, M.A., Bowman-James, K.: Enhanced anion exchange for selective sulfate extraction: overcoming the Hofmeister bias. J. Am. Chem. Soc. 130, 14386 (2008)
Moyer, B.A., Custelcean, R., Hay, B.P., Sessler, J.L., Bowman-James, K., Day, V.W., Kang, S.-O.: A case for molecular recognition in nuclear separations: sulfate separation from nuclear wastes. Inorg. Chem. 52(7), 3473–3490 (2013). doi:10.1021/ic3016832
Sessler, J.L., Gale, P.A., Cho, W.-S.: Anion receptor chemistry. The Royal Society of Chemistry, London (2006)
Qin, L., Hartley, A., Turner, P., Elmes, R.B.P., Jolliffe, K.A.: Macrocyclic squaramides: anion receptors with high sulfate binding affinity and selectivity in aqueous media. Chem. Sci. 7(7), 4563–4572 (2016). doi:10.1039/c6sc01011c
Emami Khansari, M., Mirchi, A., Pramanik, A., Johnson, C.R., Leszczynski, J., Hossain, M.A.: Remarkable hexafunctional anion receptor with operational urea-based inner cleft and thiourea-based outer cleft: novel design with high-efficiency for sulfate binding. Sci. Rep. 7(1), 6032 (2017). doi:10.1038/s41598-017-05831-x
He, Q., Kelliher, M., Bähring, S., Lynch, V.M., Sessler, J.L.: A Bis-calix[4]pyrrole enzyme mimic that constrains two oxoanions in close proximity. J. Am. Chem. Soc. 139(21), 7140–7143 (2017). doi:10.1021/jacs.7b02329
He, Q., Peters, G.M., Lynch, V.M., Sessler, J.L.: Recognition and extraction of cesium hydroxide and carbonate using a neutral multitopic ion-pair receptor. Angew. Chem. Int. Ed. (2017). doi:10.1002/anie.201705788
Seipp, C.A., Williams, N.J., Bryantsev, V.S., Moyer, B.A.: A simple guanidinium motif for the selective binding and extraction of sulfate. Sep. Sci. Technol. (2017). doi:10.1080/01496395.2017.1318922
Gale, P.A.: From anion receptors to transporters. Acc. Chem. Res. 44, 216 (2011)
Lang, C., Mohite, A., Deng, X., Yang, F., Dong, Z., Xu, J., Liu, J., Keinan, E., Reany, O.: Semithiobambus[6]uril is a transmembrane anion transporter. Chem. Commun. 53(54), 7557–7560 (2017). doi:10.1039/c7cc04026a
Havel, V., Babiak, M., Sindelar, V.: Modulation of bambusuril anion affinity in water. Chem. Eur. J. 23(37), 8963–8968 (2017). doi:10.1002/chem.201701316
Cornes, S.P., Sambrook, M.R., Beer, P.D.: Selective perrhenate recognition in pure water by halogen bonding and hydrogen bonding alpha-cyclodextrin based receptors. Chem. Commun. 53(27), 3866–3869 (2017). doi:10.1039/c7cc01605k
Acknowledgements
The authors gratefully acknowledge financial support from Max Buchner Foundation (MBFSt 3558).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wenzel, M., Weigand, J.J. Recent advances in anion recognition. J Incl Phenom Macrocycl Chem 89, 247–251 (2017). https://doi.org/10.1007/s10847-017-0756-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-017-0756-y