Abstract
Background
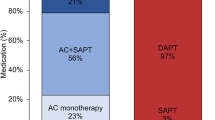
Data on antithrombotic therapy after percutaneous left atrial appendage occlusion (LAAO) is scarce and no randomized evaluation has been performed to demonstrate what is the best antithrombotic strategy. Up to date, different antithrombotic regimens with variable durations are currently used. In fact, the use of oral anticoagulation (OAC) or dual antiplatelet therapy (DAPT) with aspirin and clopidogrel during the initial phase (∓ 3 months post-LAAO) has been proposed as valid strategies. However, antiplatelet and OAC therapies have never been compared in a randomized study after left atrial appendage closure (LAAC). The purpose of the present study is to ascertain an optimal antithrombotic strategy after LAAC in terms of safety and efficacy. The study will compare a novel OAC (NOAC) with a highly safety profile like apixaban 5 mg/12 h or 2.5 mg/12 h (after dose adjustment or in high-risk patients) with standard antiplatelet therapy with DAPT. The aim of the study was to compare a strategy of anticoagulation with apixaban 5 mg/2.5 mg bid to the current standard of care (DAPT with aspirin and clopidogrel) after LAAO in patients with non-valvular atrial fibrillation (AF).
Methods
This is a phase IV multicenter randomized, open-label, controlled trial comparing the efficacy and safety of apixaban vs. DAPT after LAAO, both for 3 months. The primary endpoint is a combined endpoint of death, myocardial infarction, stroke, thromboembolic complications, and major or significant bleeding at 3 months of follow-up. Approximately 160 subjects will be enrolled and followed 12 months from randomization.
Conclusions
Considering the high risk of both thromboembolic and hemorrhagic events of patients undergoing LAAO, establishment of an appropriate antithrombotic therapy in terms of efficacy and safety after LAAO is of vital importance.
Trial registration
EudraCT number: 2018-001013-32

Similar content being viewed by others
References
GYH L, Collet JP, Caterina R, et al. Antithrombotic therapy in atrial fibrillation associated with valvular heart disease: a joint consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardi-ology Working Group on Thrombosis, endorsed by the ESC Working Group on Valvular Heart Disease, Cardiac Arrhythmia Society of Southern Africa (CASSA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), South African Heart (SA Heart) Association and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLEACE). Europace. 2017;19:1757–8.
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51.
Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17.
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91.
Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104.
Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5:615–21.
Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–47.
Meier B, Blaauw Y, Khattab AA, Lewalter T, Sievert H, Tondo C, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. Europace. 2014;16:1397–416.
Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–42.
Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12.
Lam YY, Yip GW, Yu CM, Chan WW, Cheng BC, Yan BP, et al. Left atrial appendage closure with Amplatzer cardiac plug for stroke prevention in atrial fibrillation: initial Asia-Pacific experience. Catheter Cardiovasc Interv. 2011;79:794–800.
Park JW, Bethencourt A, Sievert H, Santoro G, Meier B, Walsh K, et al. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv. 2011;77:700–6.
Reddy VY, Möbius-Winkler S, Miller MA, Neuzil P, Schuler G, Wiebe J, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix feasibility study with Watchman left atrial appendage closure technology). J Am Coll Cardiol. 2013;61:2551–6.
López-Mínguez JR, Eldoayen-Gragera J, González-Fernández R, Fernández-Vegas C, Fuentes-Cañamero ME, Millán-Nuñez V, et al. Resultados inmediatos y a más de un año en 35 pacientes consecutivos a los que se realiza cierre de orejuela izquierda con el dispositivo Amplatzer Cardiac Plug. Rev Esp Cardiol. 2013;66:90–7. https://doi.org/10.1016/j.jcin.2018.11.034.
Saw J, Nielsen-Kudsk JE, Bergmann M, Daniels MJ, Tzikas A, Reisman M, et al. Antithrombotic therapy and device-related thrombosis following endovascular left atrial appendage closure. J Am Coll Cardiol Intv. 2019;12:1067–76.
Tzikas A, Shakir S, Gafoor S, Omran H, Berti S, Santoro G, et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER cardiac plug. EuroIntervention. 2016;11:1170–9.
Freixa X, Tzikas A, Sobrino A, Chan J, Basmadjian AJ, Ibrahim R. Left atrial appendage closure with the Amplatzer cardiac plug: impact of shape and device sizing on follow-up leaks. Int J Cardiol. 2013;168(2):1023–7.
Freixa X, Abualsaud A, Chan J, Nosair M, Tzikas A, Garceau P, et al. Left atrial appendage occlusion: initial experience with the Amplatzer Amulet. Int J Cardiol. 2014;174(3):492–6.
Boersma LV, Ince H, Kische S, et al. for the EWOLUTION Investigators. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-year follow-up outcome data of the EWOLUTION trial. Heart Rhythm. 2017;14:1302–8.
Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–54.
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47.
Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41:2731–8.
Pisters R, Lane DA, Nieuwlaat R, De Vos CB, Crijns HJGM, Lip GYH. A novel userfriendly score (HAS-BLED) to assess one-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–100.
Dukkipati SR, Kar S, Holmes DR, Doshi SK, Swarup V, Gibson DN, et al. Device-related thrombus after left atrial appendage closure. Circulation. 2018;138:874–85. https://doi.org/10.1161/CIRCULATIONAHA.118.035090.
Fauchier L, Cinaud A, Brigadeau F, Lepillier A, Pierre B, Abbey S, et al. Device-related thrombosis after percutaneous left atrial appendage occlusion for atrial fibrillation. J Am Coll Cardiol. 2018;71:1528–36. https://doi.org/10.1016/j.jacc.2018.01.076.
Urena M, Rodés-Cabau J, Freixa X, Saw J, Webb JG, Freeman M, et al. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J Am Coll Cardiol. 2013;62(2):96–102.
Vidal-Jordana A, Barroeta-Espar I, Sáinz Pelayo MP, Mateo J, Delgado-Mederos R, Martí-Fàbregas J. Intracerebral hemorrhage in anticoagulated patients: what do we do afterwards?. Neurologia. 2012;(3):136–42. https://doi.org/10.1016/j.nrl.2011.04.020.
Healey JS, Hart RG, Pogue J, Pfeffer MA, Hohnloser SH, De Caterina R, et al. Risks and benefits of oral anticoagulation compared with clopidogrel plus aspirin in patients with atrial fibrillation according to stroke risk: the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events (ACTIVE-W). Stroke. 2008;39(5):1482–6. https://doi.org/10.1161/STROKEAHA.107.500199.
Kawakami T, Kobayakawa H, Ohno H, Tanaka N, Ishihara H. Resolution of left atrial appendage thrombus with apixaban. Thromb J. 2013;11(1):26. https://doi.org/10.1186/1477-9560-11-26.
Vaquerizo BS, M. Left atrial appendage thrombus resolution with reduced dose apixaban. J Atrial Fibrillation. 2015;8:28–30.
Flores-Umanzor E, Cepas-Guillen P, Sanchis L, et al. Device related thrombosis after left atrial appendage occlusion: does thrombus location always predicts its origin? [published online ahead of print, 2020 Jul 23]. J Interv Card Electrophysiol. 2020. https://doi.org/10.1007/s10840-020-00819-6.
Lempereur M, Aminian A, Freixa X, Gafoor S, Kefer J, Tzikas A, et al. Device-associated thrombus formation after left atrial appendage occlusion: a systematic review of events reported with the Watchman, the Amplatzer cardiac plug and the Amulet. Catheter Cardiovasc Interv. 2017;90(5):E111–21. https://doi.org/10.1002/ccd.26903.
Freixa X, Scalone G, Martín-Yuste V, Vidal B. Large protruding thrombus over left atrial appendage occlusion device successfully treated with apixaban. Eur Heart J. 2015;36(23):1427. https://doi.org/10.1093/eurheartj/ehv081.
Funding
The ADALA study is funded by a competitive grant from Bristol Myers Squibb/Pfizer Alliance, within the European Thrombosis Investigator–Initiated Research Program (ERISTA). Eduardo Flores-Umanzor received an Interventional Cardiology Scholarship from Dr. Alfonso Medina of the Spanish Society of Cardiology. The funders had no role in the design and conduct of the study including collection, management, analysis, and interpretation of the data.
Author information
Authors and Affiliations
Contributions
All authors had access to the data and played a role in writing this manuscript.
Corresponding author
Ethics declarations
The study was approved by the Ethics Committee of each center and adhered to the principles outlined in the Declaration of Helsinki.
Conflict of interest
XF, IC, and DA are proctors for Abbott Medical.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Flores-Umanzor, E.J., Cepas-Guillen, P.L., Arzamendi, D. et al. Rationale and design of a randomized clinical trial to compare two antithrombotic strategies after left atrial appendage occlusion: double antiplatelet therapy vs. apixaban (ADALA study). J Interv Card Electrophysiol 59, 471–477 (2020). https://doi.org/10.1007/s10840-020-00884-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-020-00884-x