Abstract
Purpose
This study was designed to determine if DMO limits in vitro development of aneuploid-enriched mouse embryos by activating a Trp53-dependent mechanism.
Methods
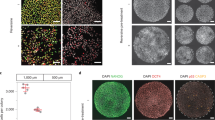
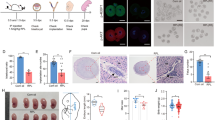
Mouse cleavage-stage embryos were treated with reversine to induce aneuploidy or vehicle to generate controls, and then cultured in media supplemented with DMO to reduce the pH of the culture media. Embryo morphology was assessed by phase microscopy. Cell number, mitotic figures, and apoptotic bodies were revealed by staining fixed embryos with DAPI. mRNA levels of Trp53, Oct-4, and Cdx2 were monitored by quantitative polymerase chain reactions (qPCRs). The effect of Trp53 on the expression of Oct-4 and Cdx2 was assessed by depleting Trp53 using Trp53 siRNA.
Results
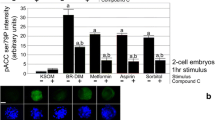
Aneuploid-enriched late-stage blastocysts were morphologically indistinguishable from control blastocysts but had fewer cells and reduced mRNA levels of Oct-4 and Cdx2. Adding 1 mM DMO to the culture media during the 8-cell to blastocyst transition reduced the formation of aneuploid-enriched late-stage blastocysts but not control blastocysts and further suppressed the levels of Oct-4 and Cdx2 mRNA. Trp53 RNA levels in aneuploid-enriched embryos that were exposed to DMO were > twofold higher than controls, and Trp53 siRNA levels reduced the levels of Trp53 and increased levels of Oct-4 and Cdx2 mRNA by > twofold.
Conclusion
These studies suggest that the development of morphologically normal aneuploid-enriched mouse blastocysts can be inhibited by adding low amounts of DMO to the culture media, which results in elevated levels of Trp53 mRNA that suppresses Oct-4 and Cdx2 expression.





Similar content being viewed by others
Data Availability
All the data from this study is available in the supplemental tables. As such all the data is available to the public.
References
McCoy RC. Mosaicism in preimplantation human embryos: when chromosomal abnormalities are the norm. Trends Genet. 2017;33:448–63.
Harton GL, Cinnioglu C, Fiorentino F. Current experience concerning mosaic embryos diagnosed during preimplantation genetic screening. Fertil Steril. 2017;107:1113–9.
Kahraman S, Cetinkaya M, Yuksel B, Yesil M, Pirkevi CC. The birth of a baby with mosaicism resulting from a known mosaic embryo transfer: a case report. Hum Reprod. 2020;35:727–33.
Treff NR, Marin D. The, “mosaic” embryo: misconceptions and misinterpretations in preimplantation genetic testing for aneuploidy. Fertil Steril. 2021;116:1205–11.
Bolton H, Graham SJ, Van der Aa N, Kumar P, Theunis K, Fernandez Gallardo E, et al. Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploid cells and normal developmental potential. Nat Commun. 2016;7:11165.
Singla S, Iwamoto-Stohl LK, Zhu M, Zernicka-Goetz M. Autophagy-mediated apoptosis eliminates aneuploid cells in a mouse model of chromosome mosaicism. Nat Commun. 2020;11:2958.
Petropoulos S, Edsgard D, Reinius B, Deng Q, Panula SP, Codeluppi S, et al. Single-cell RNA-seq reveals lineage and X chromosome dynamics in human preimplantation embryos. Cell. 2016;165:1012–26.
Yang M, Rito T, Metzger J, Naftaly J, Soman R, Hu J, et al. Author correction: depletion of aneuploid cells in human embryos and gastruloids. Nat Cell Biol. 2021;23:1212.
Zhou F, Wang R, Yuan P, Ren Y, Mao Y, Li R, et al. Reconstituting the transcriptome and DNA methylome landscapes of human implantation. Nature. 2019;572:660–4.
Mantikou E, Wong KM, Repping S, Mastenbroek S. Molecular origin of mitotic aneuploidies in preimplantation embryos. Biochim Biophys Acta. 2012;1822:1921–30.
Dawar S, Lim Y, Puccini J, White M, Thomas P, Bouchier-Hayes L, et al. Caspase-2-mediated cell death is required for deleting aneuploid cells. Oncogene. 2017;36:2704–14.
Vitale I, Manic G, Castedo M, Kroemer G. Caspase 2 in mitotic catastrophe: the terminator of aneuploid and tetraploid cells. Mol Cell Oncol. 2017;4: e1299274.
Krisher RL, Prather RS. A role for the Warburg effect in preimplantation embryo development: metabolic modification to support rapid cell proliferation. Mol Reprod Dev. 2012;79:311–20.
Gardner DK, Harvey AJ. Blastocyst metabolism. Reprod Fertil Dev. 2015;27:638–54.
Brison DR, Hewitson LC, Leese HJ. Glucose, pyruvate, and lactate concentrations in the blastocoel cavity of rat and mouse embryos. Mol Reprod Dev. 1993;35:227–32.
Gardner DK. Lactate production by the mammalian blastocyst: manipulating the microenvironment for uterine implantation and invasion? BioEssays. 2015;37:364–71.
Edwards LJ, Williams DA, Gardner DK. Intracellular pH of the preimplantation mouse embryo: effects of extracellular pH and weak acids. Mol Reprod Dev. 1998;50:434–42.
Edwards LJ, Williams DA, Gardner DK. Intracellular pH of the mouse preimplantation embryo: amino acids act as buffers of intracellular pH. Hum Reprod. 1998;13:3441–8.
Baltz JM. Intracellular pH regulation in the early embryo. BioEssays. 1993;15:523–30.
Barr KJ, Garrill A, Jones DH, Orlowski J, Kidder GM. Contributions of Na+/H+ exchanger isoforms to preimplantation development of the mouse. Mol Reprod Dev. 1998;50:146–53.
FitzHarris G, Baltz JM. Regulation of intracellular pH during oocyte growth and maturation in mammals. Reproduction. 2009;138:619–27.
Gatimel N, Moreau J, Parinaud J, Leandri RD. Need for choosing the ideal pH value for IVF culture media. J Assist Reprod Genet. 2020;37:1019–28.
Phillips KP, Leveille MC, Claman P, Baltz JM. Intracellular pH regulation in human preimplantation embryos. Hum Reprod. 2000;15:896–904.
Siyanov V, Baltz JM. NHE1 is the sodium-hydrogen exchanger active in acute intracellular pH regulation in preimplantation mouse embryos. Biol Reprod. 2013;88:157.
Zhao Y, Baltz JM. Bicarbonate/chloride exchange and intracellular pH throughout preimplantation mouse embryo development. Am J Physiol. 1996;271:C1512–20.
Jansen S, Esmaeilpour T, Pantaleon M, Kaye PL. Glucose affects monocarboxylate cotransporter (MCT) 1 expression during mouse preimplantation development. Reproduction. 2006;131:469–79.
Jansen S, Pantaleon M, Kaye PL. Characterization and regulation of monocarboxylate cotransporters Slc16a7 and Slc16a3 in preimplantation mouse embryos. Biol Reprod. 2008;79:84–92.
Zander-Fox DL, Mitchell M, Thompson JG, Lane M. Alterations in mouse embryo intracellular pH by DMO during culture impair implantation and fetal growth. Reprod Biomed Online. 2010;21:219–29.
White MD, Bissiere S, Alvarez YD, Plachta N. Mouse embryo compaction. Curr Top Dev Biol. 2016;120:235–58.
Brison DR, Schultz RM. Apoptosis during mouse blastocyst formation: evidence for a role for survival factors including transforming growth factor alpha. Biol Reprod. 1997;56:1088–96.
Nagy A, Gertsenstein M, Vintersten K, Behringer R. Collecting zygotes and removing cumulus cells with hyaluronidase. CSH Protoc 2006;2006.
Comisso E, Scarola M, Rosso M, Piazza S, Marzinotto S, Ciani Y, et al. OCT4 controls mitotic stability and inactivates the RB tumor suppressor pathway to enhance ovarian cancer aggressiveness. Oncogene. 2017;36:4253–66.
Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–91.
Daigneault BW, Rajput S, Smith GW, Ross PJ. Embryonic POU5F1 is required for expanded bovine blastocyst formation. Sci Rep. 2018;8:7753.
Jedrusik A, Cox A, Wicher KB, Glover DM, Zernicka-Goetz M. Maternal-zygotic knockout reveals a critical role of Cdx2 in the morula to blastocyst transition. Dev Biol. 2015;398:147–52.
Houghton FD, Thompson JG, Kennedy CJ, Leese HJ. Oxygen consumption and energy metabolism of the early mouse embryo. Mol Reprod Dev. 1996;44:476–85.
Tan TCY, Mahbub SB, Campbell JM, Habibalahi A, Campugan CA, Rose RD, et al. Non-invasive, label-free optical analysis to detect aneuploidy within the inner cell mass of the preimplantation embryo. Hum Reprod. 2021;37:14–29.
Li A, Chandrakanthan V, Chami O, O’Neill C. Culture of zygotes increases TRP53 [corrected] expression in B6 mouse embryos, which reduces embryo viability. Biol Reprod. 2007;76:362–7.
Wells D, Bermudez MG, Steuerwald N, Malter HE, Thornhill AR, Cohen J. Association of abnormal morphology and altered gene expression in human preimplantation embryos. Fertil Steril. 2005;84:343–55.
Wells D, Bermudez MG, Steuerwald N, Thornhill AR, Walker DL, Malter H, et al. Expression of genes regulating chromosome segregation, the cell cycle and apoptosis during human preimplantation development. Hum Reprod. 2005;20:1339–48.
Regin M, Spits C, Sermon K. On the origins and fate of chromosomal abnormalities in human preimplantation embryos: an unsolved riddle. Mol Hum Reprod. 2022;28(4):1–13.
Jain AK, Allton K, Iacovino M, Mahen E, Milczarek RJ, Zwaka TP, et al. p53 regulates cell cycle and microRNAs to promote differentiation of human embryonic stem cells. PLoS Biol. 2012;10: e1001268.
Liu Y, White KA, Barber DL. Intracellular pH regulates cancer and stem cell behaviors: a protein dynamics perspective. Front Oncol. 2020;10:1401.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplemental Figure 1.
The effect of reversine (5 μM) on the induction of aneuploidy as revealed by FISH analysis. The upper panel shows a typical diploid blastomere with two red dots and two green dots that represent chromosome 11q and 2q, respectively as well as examples of various aneuploid blastomeres. The table in the lower panel presents the ploidy status of all the blastomeres that were analyzed after DMSO (control) and reversine treatment as revealed by the number of 11q and 2q chromosomes present. Ploidy status for each blastomeres is indicated by a D (diploid) or A (aneuploid). (PNG 2491 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lowther, K.M., Bartolucci, A.F., Massey, R.E. et al. Supplementing culture medium with the weak acid, 5,5-dimethyl-2,4-oxazolidinedione (DMO) limits the development of aneuploid mouse embryos through a Trp53-dependent mechanism. J Assist Reprod Genet 40, 1215–1223 (2023). https://doi.org/10.1007/s10815-023-02788-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02788-x