Abstract
Purpose
To report outcome of planned oocyte cryopreservation (POC) in the first 8 years of this treatment in our center.
Methods
A retrospective study in a university-affiliated medical center.
Results
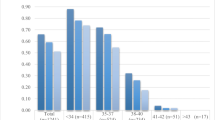
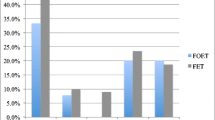
A total of 446 women underwent POC during 2011–2018. Fifty-seven (13%) women presented to use these oocytes during the study period (until June 2021). POC was performed at a mean age of 37.9 ± 2.0 (range 33–41). Age at thawing was 43.3 ± 2.1 (range 38–49). A total of 34 (60%) women transferred their oocytes for thawing at other units. Oocyte survival after thawing was significantly higher at our center than following shipping to ancillary sites (78 vs. 63%, p = 0.047). Forty-nine women completed their treatment, either depleting their cryopreserved oocytes without conceiving (36) or attaining a live birth (13)—27% live birth rate per woman. Only one of eleven women who cryopreserved oocytes aged 40 and older had a live birth using thawed oocytes.
Conclusion
Women should be advised to complete planned oocyte cryopreservation before age 40, given low success rates in women who underwent cryopreservation at advanced reproductive age. In this study, oocyte shipping was associated with lower survival rate. These findings may be relevant for women considering POC and utilization of cryopreserved oocytes.
Similar content being viewed by others
References
Ethics Committee of the American Society for Reproductive Medicine. Planned oocyte cryopreservation for women seeking to preserve future reproductive potential: an Ethics Committee opinion. Fertil Steril. 2018;110:1022–8. https://doi.org/10.1016/j.fertnstert.2018.08.027.
Cobo A, Remohí J, Chang C-C, Nagy ZP. Oocyte cryopreservation for donor egg banking. Reprod Biomed Online. 2011;23:341–6. https://doi.org/10.1016/j.rbmo.2011.05.014.
Dolmans M-M, Donnez J. Fertility preservation in women for medical and social reasons: oocytes vs ovarian tissue. Best Pract Res Clin Obstet Gynaecol. 2021;70:63–80. https://doi.org/10.1016/j.bpobgyn.2020.06.011.
Cobo A, Coello A, de Los Santos MJ, Giles J, Pellicer A, Remohí J, García-Velasco JA. Number needed to freeze: cumulative live birth rate after fertility preservation in women with endometriosis. Reprod Biomed Online. 2021;42:725–32. https://doi.org/10.1016/j.rbmo.2020.12.013.
Iussig B, Maggiulli R, Fabozzi G, Bertelle S, Vaiarelli A, Cimadomo D, et al. A brief history of oocyte cryopreservation: arguments and facts. Acta Obstet Gynecol Scand. 2019;98:550–8. https://doi.org/10.1111/aogs.13569.
Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23:139–55. https://doi.org/10.1093/humupd/dmw038.
Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online. 2009;18:769–76. https://doi.org/10.1016/s1472-6483(10)60025-9.
Dondorp W, de Wert G, Pennings G, Shenfield F, Devroey P, Tarlatzis B, et al. Oocyte cryopreservation for age-related fertility loss. Hum Reprod. 2012;27:1231–7. https://doi.org/10.1093/humrep/des029.
Johnston M, Richings NM, Leung A, Sakkas D, Catt S. A major increase in oocyte cryopreservation cycles in the USA, Australia and New Zealand since 2010 is highlighted by younger women but a need for standardized data collection. Hum Reprod. 2021;36:624–35. https://doi.org/10.1093/humrep/deaa320.
HFEA. Egg freezing in fertility treatment. Trends and figures: 2010–2016. Human fertilisation and embryology authority. 2018. https://www.hfea.gov.uk/media/2656/egg-freezing-in-fertility-treatment-trends-and-figures-2010-2016-final.pdf
Hodes-Wertz B, Druckenmiller S, Smith M, Noyes N. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril. 2013;100:1343–9. https://doi.org/10.1016/j.fertnstert.2013.07.201.
Balkenende EM, Dahhan T, van der Veen F, Repping S, Goddijn M. Reproductive outcomes after oocyte banking for fertility preservation. Reprod Biomed Online. 2018;37:425–33. https://doi.org/10.1016/j.rbmo.2018.07.005.
Gürtin ZB, Morgan L, O’Rourke D, Wang J, Ahuja K. For whom the egg thaws: insights from an analysis of 10 years of frozen egg thaw data from two UK clinics, 2008–2017. J Assist Reprod Genet. 2019;36:1069–80. https://doi.org/10.1007/s10815-019-01429-6.
Hammarberg K, Kirkman M, Pritchard N, Hickey M, Peate M, McBain J, et al. Reproductive experiences of women who cryopreserved oocytes for non-medical reasons. Hum Reprod. 2017;32:575–81. https://doi.org/10.1093/humrep/dew342.
Wennberg A-L, Schildauer K, Brännström M. Elective oocyte freezing for nonmedical reasons: a 6-year report on utilization and in vitro fertilization results from a Swedish center. Acta Obstet Gynecol Scand. 2019;98:1429–34. https://doi.org/10.1111/aogs.13673.
Wafi A, Nekkebroeck J, Blockeel C, de Munck N, Tournaye H, de Vos M. A follow-up survey on the reproductive intentions and experiences of women undergoing planned oocyte cryopreservation. Reprod Biomed Online. 2020;40:207–14. https://doi.org/10.1016/j.rbmo.2019.11.010.
Yee S, Goodman CV, Fu V, Lipton NJ, Librach CL. Parenthood desire, childbearing plans and oocyte utilization among women who previously underwent planned oocyte cryopreservation. Reprod Biomed Online. 2020. https://doi.org/10.1016/j.rbmo.2020.10.004.
Jones BP, Kasaven L, L’Heveder A, Jalmbrant M, Green J, Makki M, et al. Perceptions, outcomes, and regret following social egg freezing in the UK; a cross-sectional survey. Acta Obstet Gynecol Scand. 2020;99:324–32. https://doi.org/10.1111/aogs.13763.
Leung AQ, Baker K, Vaughan D, Shah JS, Korkidakis A, Ryley DA, et al. Clinical outcomes and utilization from over a decade of planned oocyte cryopreservation. Reprod Biomed Online. 2021;43:671–9. https://doi.org/10.1016/j.rbmo.2021.06.024.
Cobo A, García-Velasco J, Domingo J, Pellicer A, Remohí J. Elective and onco-fertility preservation: factors related to IVF outcomes. Hum Reprod. 2018;33:2222–31. https://doi.org/10.1093/humrep/dey321.
Tsafrir A, Holzer H, Miron-Shatz T, Eldar-Geva T, Gal M, Ben-Ami I, et al. ‘Why have women not returned to use their frozen oocytes?’: a 5-year follow-up of women after planned oocyte cryopreservation. Reprod Biomed Online. 2021;43:1137–45. https://doi.org/10.1016/j.rbmo.2021.08.026.
Blakemore JK, Grifo JA, DeVore SM, Hodes-Wertz B, Berkeley AS. Planned oocyte cryopreservation-10-15-year follow-up: return rates and cycle outcomes. Fertil Steril. 2021;115:1511–20. https://doi.org/10.1016/j.fertnstert.2021.01.011.
Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11:300–8. https://doi.org/10.1016/s1472-6483(10)60837-1.
The Istanbul consensus workshop on embryo assessment. proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83. https://doi.org/10.1093/humrep/der037.
OECD. Fertility rates. https://data.oecd.org/pop/fertility-rates.htm. Accessed 7 Jan 2020.
DeVore S, Noyes N, Grifo JA, Berkeley AS, Licciardi F, Goldman KN. Achieving the “ideal” family size at advanced reproductive ages through oocyte cryopreservation. J Assist Reprod Genet. 2019;36:277–82. https://doi.org/10.1007/s10815-018-1303-5.
McDonald CA, Valluzo L, Chuang L, Poleshchuk F, Copperman AB, Barritt J. Nitrogen vapor shipment of vitrified oocytes: time for caution. Fertil Steril. 2011;95:2628–30. https://doi.org/10.1016/j.fertnstert.2011.05.053.
Parmegiani L. Oocyte vitrification/storage/handling/transportation/warming, effect on survival and clinical results in donation programmes. CCE. 2017;4:34. https://doi.org/10.11138/CCE/2017.4.2.034.
The Israeli National IVF Registry. 2019 Annual Report. file:///C:/Users/Owner/Downloads/The%20Israeli%20National%20IVF%20Registry%20final%20Report%2022102019.pdf. Accessed 1 Mar 2022.
Smith ADAC, Tilling K, Nelson SM, Lawlor DA. Live-birth rate associated with repeat in vitro fertilization treatment cycles. JAMA. 2015;314:2654–62. https://doi.org/10.1001/jama.2015.17296.
Lebovitz O, Haas J, James KE, Seidman DS, Orvieto R, Hourvitz A. The expected cumulative incidence of live birth for patients starting IVF treatment at age 41 years or older. Reprod Biomed Online. 2018;37:533–41. https://doi.org/10.1016/j.rbmo.2018.08.014.
Raz N, Shalev A, Horowitz E, Weissman A, Mizrachi Y, Ganer Herman H, Raziel A. Cumulative pregnancy and live birth rates through assisted reproduction in women 44–45 years of age: is there any hope? J Assist Reprod Genet. 2018;35:441–7. https://doi.org/10.1007/s10815-017-1094-0.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsafrir, A., Ben-Ami, I., Eldar-Geva, T. et al. Clinical outcome of planned oocyte cryopreservation at advanced age. J Assist Reprod Genet 39, 2625–2633 (2022). https://doi.org/10.1007/s10815-022-02633-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02633-7