Abstract
Purpose
Adequate androgen levels are necessary for regular follicular growth, progression beyond the pre-antral stage, and prevention of follicular atresia. The main purpose of this study was to investigate whether baseline androgen levels had a predictive value on stimulation outcomes in IVF cycles. The secondary purpose was to compare the possible predictive value of androgens with that of already known markers.
Methods
The study included 91 infertile patients aged 30–45 years awaiting the first IVF cycle. All women underwent the same stimulation protocol and the same starting dose of recombinant FSH. As stimulation outcomes, the number of follicles recruited, estradiol and progesterone levels on the day of trigger, the total dose of gonadotropins administered, and the number of oocytes collected were recorded. Multiple linear regression and multivariate logistic regression were used to evaluate the significant predictive value of the variables for response to controlled ovarian stimulation (COS). By studying the reliability of different markers, an attempt was made to develop a single index with the highest predictive value.
Results
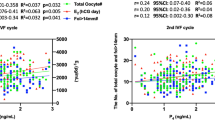
Pearson’s correlation revealed a statistically significant inverse correlation between oocytes collected and age (r = − 0.333, p < 0.001) and a positive correlation with AMH (anti-müllerian hormone) (r = 0.360, p < 0.001), antral follicle count (AFC) (r = 0.639, p < 0.001), and androstenedione (Δ4-A) (r = 0.359, p < 0.001). No significant correlation was reported with FSH (r = − 0.133, p = 0.207) and total testosterone (r = 0.180, p = 0.088). In COS good responders, the G-index (= AMH ng/mL*AFC/Δ4-A ng/dL) revealed a significantly higher level (p < 0.001) than AMH, AFC, and Δ4-A alone.
Conclusion
Baseline serum Δ4-A, presumably crucial for ensuring a regular follicular growth, is a reliable marker of ovarian response to stimulation. Since the ovarian capacity to respond to gonadotropins does not depend exclusively on the presence of follicles, we suggest a new index, the G-index, able to contemplate both the ovarian reserve and the Δ4-A level.





Similar content being viewed by others
References
Sunkara SK, et al. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. 2011;26(7):1768–74.
Verberg MF, et al. Why do couples drop-out from IVF treatment? A prospective cohort study. Hum Reprod. 2008;23(9):2050–5.
Baker VL, et al. Association of number of retrieved oocytes with live birth rate and birth weight: an analysis of 231,815 cycles of in vitro fertilization. Fertil Steril. 2015;103(4):931-938.e2.
Fauser BC, Diedrich K, Devroey P. Predictors of ovarian response: progress towards individualized treatment in ovulation induction and ovarian stimulation. Hum Reprod Update. 2008;14(1):1–14.
de Boer EJ, et al. A low number of retrieved oocytes at in vitro fertilization treatment is predictive of early menopause. Fertil Steril. 2002;77(5):978–85.
te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8(2):141–54.
Klinkert ER, et al. A poor response in the first in vitro fertilization cycle is not necessarily related to a poor prognosis in subsequent cycles. Fertil Steril. 2004;81(5):1247–53.
Broer SL, et al. Prediction of an excessive response in in vitro fertilization from patient characteristics and ovarian reserve tests and comparison in subgroups: an individual patient data meta-analysis. Fertil Steril. 2013;100(2):420-9.e7.
Broekmans FJ, et al. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685–718.
La Marca A, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update. 2010;16(2):113–30.
Verhagen TE, et al. The accuracy of multivariate models predicting ovarian reserve and pregnancy after in vitro fertilization: a meta-analysis. Hum Reprod Update. 2008;14(2):95–100.
Ferraretti AP, et al. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616–24.
Kwee J, et al. Comparison of endocrine tests with respect to their predictive value on the outcome of ovarian hyperstimulation in IVF treatment: results of a prospective randomized study. Hum Reprod. 2003;18(7):1422–7.
Yang MY, Fortune JE. Testosterone stimulates the primary to secondary follicle transition in bovine follicles in vitro. Biol Reprod. 2006;75(6):924–32.
Walters KA, Allan CM, Handelsman DJ. Androgen actions and the ovary. Biol Reprod. 2008;78(3):380–9.
Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24(7):1393–403.
Nielsen ME, et al. In human granulosa cells from small antral follicles, androgen receptor mRNA and androgen levels in follicular fluid correlate with FSH receptor mRNA. Mol Hum Reprod. 2011;17(1):63–70.
Burger HG. Androgen production in women. Fertil Steril. 2002;77(Suppl 4):S3-5.
Meldrum DR, et al. Role of decreased androgens in the ovarian response to stimulation in older women. Fertil Steril. 2013;99(1):5–11.
van der Stege JG, et al. Decreased androgen concentrations and diminished general and sexual well-being in women with premature ovarian failure. Menopause. 2008;15(1):23–31.
Gleicher N, et al. Hypoandrogenism in association with diminished functional ovarian reserve. Hum Reprod. 2013;28(4):1084–91.
Norman RJ. Hyperandrogenaemia and the ovary. Mol Cell Endocrinol. 2002;191(1):113–9.
Pigny P, et al. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88(12):5957–62.
Sen A, et al. Endocrine autoimmune diseases and female infertility. Nat Rev Endocrinol. 2014;10(1):37–50.
Frattarelli JL, Peterson EH. Effect of androgen levels on in vitro fertilization cycles. Fertil Steril. 2004;81(6):1713–4.
Frattarelli JL, Gerber MD. Basal and cycle androgen levels correlate with in vitro fertilization stimulation parameters but do not predict pregnancy outcome. Fertil Steril. 2006;86(1):51–7.
Qin Y, et al. Association of basal serum testosterone levels with ovarian response and in vitro fertilization outcome. Reprod Biol Endocrinol. 2011;9:9.
Sun B, et al. Basal serum testosterone levels correlate with ovarian response but do not predict pregnancy outcome in non-PCOS women undergoing IVF. J Assist Reprod Genet. 2014;31(7):829–35.
Sullivan GM, Feinn R. Using effect size—or why the P value is not enough. J Grad Med Educ. 2012;4(3):279–82.
Broekmans FJ, et al. The antral follicle count: practical recommendations for better standardization. Fertil Steril. 2010;94(3):1044–51.
Abbara A, et al. Follicle size on day of trigger most likely to yield a mature oocyte. Front Endocrinol (Lausanne). 2018;9:193.
Ji J, et al. The optimum number of oocytes in IVF treatment: an analysis of 2455 cycles in China. Hum Reprod. 2013;28(10):2728–34.
Ozyurek ES, Yoldemir T, Artar G. Androstenedione response to recombinant human FSH is the most valid predictor of the number of selected follicles in polycystic ovarian syndrome: (a case-control study). J Ovarian Res. 2017;10(1):34.
Menet MC, et al. rFSH in medically assisted procreation: evidence for ovarian follicular hyperplasia and interest of mass spectrometry to measure 17-hydroxyprogesterone and Δ4-androstenedione in serum. Mol Cell Endocrinol. 2017;450:105–12.
Walters KA, Handelsman DJ. Role of androgens in the ovary. Mol Cell Endocrinol. 2018;465:36–47.
Murray AA, et al. Effect of androgens on the development of mouse follicles growing in vitro. J Reprod Fertil. 1998;113(1):27–33.
Wang H, et al. Effect of adrenal and ovarian androgens on type 4 follicles unresponsive to FSH in immature mice. Endocrinology. 2001;142(11):4930–6.
Gleicher N, et al. The importance of adrenal hypoandrogenism in infertile women with low functional ovarian reserve: a case study of associated adrenal insufficiency. Reprod Biol Endocrinol. 2016;14:23.
Casson PR, et al. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod. 2000;15(10):2129–32.
Barad D, Gleicher N. Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Hum Reprod. 2006;21(11):2845–9.
Gleicher N, Barad DH. Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR). Reprod Biol Endocrinol. 2011;9(1):67.
Mamas L, Mamas E. Premature ovarian failure and dehydroepiandrosterone. Fertil Steril. 2009;91(2):644–6.
Sunkara SK, Coomarasamy A. Androgen pretreatment in poor responders undergoing controlled ovarian stimulation and in vitro fertilization treatment. Fertil Steril. 2011;95(8):e73-4 (author reply e75).
Barad D, Brill H, Gleicher N. Update on the use of dehydroepiandrosterone supplementation among women with diminished ovarian function. J Assist Reprod Genet. 2007;24(12):629–34.
Yeung TW, et al. A randomized double-blinded placebo-controlled trial on the effect of dehydroepiandrosterone for 16 weeks on ovarian response markers in women with primary ovarian insufficiency. J Clin Endocrinol Metab. 2013;98(1):380–8.
Narkwichean A, et al. Efficacy of dehydroepiandrosterone (DHEA) to overcome the effect of ovarian ageing (DITTO): a proof of principle double blinded randomized placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2017;218:39–48.
Ferrario M, et al. Ovarian and adrenal androgens may be useful markers to predict oocyte competence and embryo development in older women. Gynecol Endocrinol. 2015;31(2):125–30.
Author information
Authors and Affiliations
Contributions
E.G. conceived the presented idea and managed the project. V.G., G.M., G.Ma, E.Gu, and J.R. contributed to acquisition of data. A.M.M. and P.S. helped supervise the project. L.G. helped analyze the data. V.G., L.G., and E.G. drafted and finalized the manuscript. All authors agreed with the submission of the manuscript and gave approval to the final version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garzia, E., Galiano, V., Guarnaccia, L. et al. Basal serum level of Δ4-androstenedione reflects the ovaries’ ability to respond to stimulation in IVF cycles: setting up a new reliable index of both ovarian reserve and response. J Assist Reprod Genet 39, 1917–1926 (2022). https://doi.org/10.1007/s10815-022-02546-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02546-5