Abstract
Purpose
To investigate whether fatty acid changes in granulosa cells (GCs) underly the pathogenic mechanisms of diminished ovarian reserve (DOR).
Methods
GCs were obtained from patients with DOR (n = 70) and normal ovarian reserve (NOR, n = 70). Analysis of fatty acids changes in GCs was then analyzed.
Results
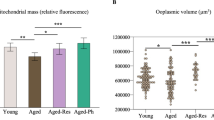
Patients with DOR had significantly lower levels of antral follicle count and anti-Mullerian hormone and higher levels of follicle-stimulating hormone compared with NOR patients (P < 0.001). The good-quality embryo rate was notably decreased in DOR patients (51.99 vs 39.52%, P < 0.05). A total of 15 significantly decreased fatty acids in GCs from patients with DOR. The ATP levels were markedly lower in DOR patients than in NOR patients (39.07 ± 12.89 vs 23.21 ± 13.69%, P < 0.05). Mitochondrial membrane potential decreased in DOR patients (P < 0.01). In GCs from DOR patients, the β-oxidation genes (HADHA and ACSL) and DNA repair genes (PRKDC and RAD50) were significantly downregulated (P < 0.05). The γH2AX foci/nucleus ratio in DOR patients markedly increased relative to that of NOR patients (0.31 ± 0.03 vs 0.87 ± 0.07, P < 0.001). Meanwhile, the apoptosis rate of GCs was significantly higher in DOR patients (6.43 ± 2.11 vs 48.06 ± 6.72%, P < 0.01).
Conclusion
GC apoptosis resulting from the decrease of fatty acids, and associated with reduced ATP production and DNA damage, may contribute to the pathogenic mechanisms responsible for DOR.




Similar content being viewed by others
References
Greene AD, Patounakis G, Segars JH. Genetic associations with diminished ovarian reserve: a systematic review of the literature. J Assist Reprod Genet. 2014;31(8):935–46.
Pal L, Bevilacqua K, Santoro NF. Chronic psychosocial stressors are detrimental to ovarian reserve: a study of infertile women. J Psychosom Obstet Gynaecol. 2010;31(3):130–9.
Mutlu MF, Erdem A. Evaluation of ovarian reserve in infertile patients. J Turk Ger Gynecol Assoc. 2012;13(3):196–203.
Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci. 2004;82–83:431–46.
Dunning KR, Russell DL, Robker RL. Lipids and oocyte developmental competence: the role of fatty acids and β-oxidation. Reproduction. 2014;148(1):R15-27.
Downs SM, Mosey JL, Klinger J. Fatty acid oxidation and meiotic resumption in mouse oocytes. Mol Reprod Dev. 2009;76(9):844–53.
Sturmey RG, O’Toole PJ, Leese HJ. Fluorescence resonance energy transfer analysis of mitochondrial:lipid association in the porcine oocyte. Reproduction. 2006;132(6):829–37.
Dunning KR, et al. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol Reprod. 2010;83(6):909–18.
Zhao Z, et al. The mRNA expression signature and prognostic analysis of multiple fatty acid metabolic enzymes in clear cell renal cell carcinoma. J Cancer. 2019;10(26):6599–607.
Bradley J, Swann K. Mitochondria and lipid metabolism in mammalian oocytes and early embryos. Int J Dev Biol. 2019;63(3–4–5):93–103.
Dunning KR, et al. Increased beta-oxidation and improved oocyte developmental competence in response to l-carnitine during ovarian in vitro follicle development in mice. Biol Reprod. 2011;85(3):548–55.
Nagata S, et al. Effect of aging on mitochondria and metabolism of bovine granulosa cells. J Reprod Dev. 2020;66(6):547–54.
Ge H, et al. The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence. Mol Reprod Dev. 2012;79(6):392–401.
Babayev E, Seli E. Oocyte mitochondrial function and reproduction. Curr Opin Obstet Gynecol. 2015;27(3):175–81.
de Barros TT, et al. DNA damage is inversely associated to blood levels of DHA and EPA fatty acids in Brazilian children and adolescents. Food Funct. 2020;11(6):5115–21.
Zhang D, et al. Increased DNA damage and repair deficiency in granulosa cells are associated with ovarian aging in rhesus monkey. J Assist Reprod Genet. 2015;32(7):1069–78.
Qian M, et al. Boosting ATM activity alleviates aging and extends lifespan in a mouse model of progeria. Elife. 2018;7.e348360
Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308(5721):551–4.
Goodwin JF, Knudsen KE. Beyond DNA repair: DNA-PK function in cancer. Cancer Discov. 2014;4(10):1126–39.
Roos WP, Kaina B. DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013;332(2):237–48.
Fan Y, et al. Apoptosis of mural granulosa cells is increased in women with diminished ovarian reserve. J Assist Reprod Genet. 2019;36(6):1225–35.
Cil AP, et al. Assessment of ovarian reserve and Doppler characteristics in patients with multiple sclerosis using immunomodulating drugs. J Turk Ger Gynecol Assoc. 2009;10(4):213–9.
Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114(6):1151-1157.
Li R, Albertini DF. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol. 2013;14(3):141–52.
Huang R, et al. Alterations of polyunsaturated fatty acid metabolism in ovarian tissues of polycystic ovary syndrome rats. J Cell Mol Med. 2018;22(7):3388–96.
Sawyer BT, et al. Targeting fatty acid oxidation to promote anoikis and inhibit ovarian cancer progression. Mol Cancer Res. 2020;18(7):1088–98.
McKeegan PJ, Sturmey RG. The role of fatty acids in oocyte and early embryo development. Reprod Fertil Dev. 2011;24(1):59–67.
O’Gorman A, et al. Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence. Reproduction. 2013;146(4):389–95.
Dubeibe Marin DF, et al. Importance of lipid metabolism on oocyte maturation and early embryo development: can we apply what we know to buffalo? Anim Reprod Sci. 2019;211:106220.
Dunning KR, et al. Regulation of fatty acid oxidation in mouse cumulus-oocyte complexes during maturation and modulation by PPAR agonists. PLoS One. 2014;9(2):e87327.
Ohata K, et al. Effects of fatty acid supplementation during vitrification and warming on the developmental competence of mouse, bovine and human oocytes and embryos. Reprod Biomed Online. 2021;43(1):14–25.
Wang HW, et al. Activity of long-chain acyl-CoA synthetase is required for maintaining meiotic arrest in Xenopus laevis. Biol Reprod. 2012;87(3):74.
Igarashi H, et al. Aged mouse oocytes fail to readjust intracellular adenosine triphosphates at fertilization. Biol Reprod. 2005;72(5):1256–61.
Sullivan EM, et al. Mechanisms by which dietary fatty acids regulate mitochondrial structure-function in health and disease. Adv Nutr. 2018;9(3):247–62.
Dumesic DA, et al. Cumulus cell mitochondrial resistance to stress in vitro predicts oocyte development during assisted reproduction. J Clin Endocrinol Metab. 2016;101(5):2235–45.
Xu G, et al. Cadmium induces apoptosis of human granulosa cell line KGN via mitochondrial dysfunction-mediated pathways. Ecotoxicol Environ Saf. 2021;220:112341.
Lee KS, et al. Cumulus cells apoptosis as an indicator to predict the quality of oocytes and the outcome of IVF-ET. J Assist Reprod Genet. 2001;18(9):490–8.
Banim PJ, et al. Dietary oleic acid is inversely associated with pancreatic cancer - data from food diaries in a cohort study. Pancreatology. 2018;18(6):655–60.
Winship AL, et al. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing. Hum Reprod Update. 2018;24(2):119–34.
Stringer JM, et al. The capacity of oocytes for DNA repair. Cell Mol Life Sci. 2018;75(15):2777–92.
Bedoschi GM, Navarro PA, Oktay KH. Novel insights into the pathophysiology of chemotherapy-induced damage to the ovary. Panminerva Med. 2019;61(1):68–75.
Glamoclija V, et al. Apoptosis and active caspase-3 expression in human granulosa cells. Fertil Steril. 2005;83(2):426–31.
Stoffel W, et al. Dietary ω3-and ω6-polyunsaturated fatty acids reconstitute fertility of juvenile and adult Fads2-deficient mice. Mol Metab. 2020;36:100974.
Zarezadeh R, et al. Fatty acids of follicular fluid phospholipids and triglycerides display distinct association with IVF outcomes. Reprod Biomed Online. 2021;42(2):301–9.
Yang Y, et al. Metabolic changes of maternal uterine fluid, uterus, and plasma during the peri-implantation period of early pregnancy in mice. Reprod Sci. 2020;27(2):488–502.
Karaşahin T. The effect of oleic and linoleic acid addition to the culture media on bovine embryonic development following vitrification. Pol J Vet Sci. 2019;22(4):661–6.
Haggarty P, et al. Fatty acid metabolism in human preimplantation embryos. Hum Reprod. 2006;21(3):766–73.
Hohos NM, et al. Fat-1 transgene is associated with improved reproductive outcomes. Endocrinology. 2018;159(12):3981–92.
Sun X, et al. Research on serum metabolomics of ovariectomized rats and intervention effect of Cuscuta chinensis on metabolic pattern. J Pharm Biomed Anal. 2021;195:113847.
Wu TC, Wang L, Wan YJ. Expression of estrogen receptor gene in mouse oocyte and during embryogenesis. Mol Reprod Dev. 1992;33(4):407–12.
Panagiotopoulos AA, et al. Eicosanoids in prostate cancer. Cancer Metastasis Rev. 2018;37(2–3):237–43.
Khajeh M, et al. Potential role of polyunsaturated fatty acids, with particular regard to the signaling pathways of arachidonic acid and its derivatives in the process of maturation of the oocytes: contemporary review. Biomed Pharmacother. 2017;94:458–67.
Hoyos-Marulanda V, et al. Effects of polyunsaturated fatty acids on the development of pig oocytes in vitro following parthenogenetic activation and on the lipid content of oocytes and embryos. Anim Reprod Sci. 2019;205:150–5.
Jahangirifar M, et al. Dietary fatty acid intakes and the outcomes of assisted reproductive technique in infertile women. J Reprod Infertil. 2021;22(3):173–83.
Acknowledgements
This research was supported by the National Natural Science Foundation of China(No.81960278), the Outstanding Youth Funds of Science and Technology Department of Gansu Province (No.20JR5RA371)(No.20JR10RA701), and Fundamental Research Funds for the Central Universities (No.lzujbky-2021-kd38)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the ethics committee of the First Hospital of Lanzhou University (LDYYLL2019-44).
Consent to participate
Available.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, Z., Fan, Q., Zhu, Q. et al. Decreased fatty acids induced granulosa cell apoptosis in patients with diminished ovarian reserve. J Assist Reprod Genet 39, 1105–1114 (2022). https://doi.org/10.1007/s10815-022-02462-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02462-8