Abstract
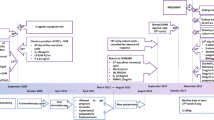
With the increased rate of stable remission after gonadotoxic cancer treatment, new methods of fertility preservation are required in order to provide the best possible care for oncological patients. Here, we report an original case of euploid blastocyst cryopreservation after in vitro maturation of ovarian tissue oocytes (OTO IVM). Thirty-three oocytes were obtained from the ovarian tissue after ovariectomy in the breast cancer patient. Six out of 12 matured oocytes fertilized successfully and 3 blastocysts were formed. Genetic investigation for mutations associated with this type of malignancy found that the patient is not a carrier. Preimplantation genetic testing was performed only for aneuploidies and found all 3 blastocysts to be euploid and suitable for embryo transfer. Our study showed that the ovarian tissue oocytes matured in vitro have the potential for euploid blastocyst formation after ICSI which could be screened for aneuploidies and inherited mutations and then be vitrified in order to provide the best fertility preservation strategy for women with cancer.


Similar content being viewed by others
References
Shah NM, Scott DM, Kandagatla P, Moravek MB, Cobain EF, Burness ML, et al. Young women with breast cancer: fertility preservation options and management of pregnancy-associated breast cancer. Ann Surg Oncol. 2019 [Internet]. [cited 2019 Feb 13]; Available from: http://link.springer.com/10.1245/s10434-019-07156-7;26:1214–24.
Ruddy KJ, Gelber S, Ginsburg ES, Schapira L, Abusief ME, Meyer ME, et al. Menopausal symptoms and fertility concerns in premenopausal breast cancer survivors: a comparison to age- and gravidity-matched controls. Menopause. 2011;18:105–8.
Christinat A, Pagani O. Fertility after breast cancer. Maturitas. 2012;73:191–6.
Medrano JV, Andrés MDM, García S, Herraiz S, Vilanova-Pérez T, Goossens E, et al. Basic and clinical approaches for fertility preservation and restoration in cancer patients. Trends Biotechnol. 2018;36:199–215.
The Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. Cancer Spectrum Knowledge Environment. 2002;94:606–16.
Sonmezer M. Fertility preservation in female patients. Hum Reprod Update. 2004;10:251–66.
Pacheco F, Oktay K. Current success and efficiency of autologous ovarian transplantation: a meta-analysis. Reprod Sci. 2017;24:1111–20.
Yin H, Jiang H, Kristensen SG, Andersen CY. Vitrification of in vitro matured oocytes collected from surplus ovarian medulla tissue resulting from fertility preservation of ovarian cortex tissue. J Assist Reprod Genet. 2016;33:741–6.
Revel A. Oocyte collection during cryopreservation of the ovarian cortex. Fertil Steril. 2003;79:1237–9.
Fasano G, Moffa F, Dechène J, Englert Y, Demeestere I. Vitrification of in vitro matured oocytes collected from antral follicles at the time of ovarian tissue cryopreservation. Reprod Biol Endocrinol. 2011;9:150.
Hourvitz A, Yerushalmi GM, Maman E, Raanani H, Elizur S, Brengauz M, et al. Combination of ovarian tissue harvesting and immature oocyte collection for fertility preservation increases preservation yield. Reprod BioMed Online. 2015;31:497–505.
Segers I, Mateizel I, Van Moer E, Smitz J, Tournaye H, Verheyen G, et al. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J Assist Reprod Genet. 2015;32:1221–31.
Park CW, Lee SH, Yang KM, Lee IH, Lim KT, Lee KH, et al. Cryopreservation of in vitro matured oocytes after ex vivo oocyte retrieval from gynecologic cancer patients undergoing radical surgery. Clin Exp Reprod Med. 2016;43:119.
Fadini R, Dal Canto M, Mignini Renzini M, Milani R, Fruscio R, Cantù MG, et al. Embryo transfer following in vitro maturation and cryopreservation of oocytes recovered from antral follicles during conservative surgery for ovarian cancer. J Assist Reprod Genet. 2012;29:779–81.
Prasath EB, Chan MLH, Wong WHW, Lim CJW, Tharmalingam MD, Hendricks M, et al. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29:276–8.
Uzelac PS, Delaney AA, Christensen GL, Bohler HCL, Nakajima ST. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil Steril. 2015;104:1258–60.
Schmidt KLT. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod. 2003;18:1158–64.
Escribá M-J, Grau N, Escrich L, Novella-Maestre E, Sánchez-Serrano M. Spontaneous in vitro maturation of oocytes prior to ovarian tissue cryopreservation in natural cycles of oncologic patients. J Assist Reprod Genet. 2012;29:1261–5.
Isachenko E, Rahimi G, Isachenko V, Nawroth F. In-vitro maturation of germinal-vesicle oocytes and cryopreservation in metaphase I/II: a possible additional option to preserve fertility during ovarian tissue cryopreservation. Reprod BioMed Online. 2004;8:553–7.
Kedem A, Yerushalmi GM, Brengauz M, Raanani H, Orvieto R, Hourvitz A, et al. Outcome of immature oocytes collection of 119 cancer patients during ovarian tissue harvesting for fertility preservation. J Assist Reprod Genet. 2018;35:851–6.
Huang JYJ, Tulandi T, Holzer H, Tan SL, Chian R-C. Combining ovarian tissue cryobanking with retrieval of immature oocytes followed by in vitro maturation and vitrification: an additional strategy of fertility preservation. Fertil Steril. 2008;89:567–72.
The Vienna consensus: report of an expert meeting on the development of ART laboratory performance indicators. Reprod BioMed Online. 2017;35:494–510.
Shirasawa H, Terada Y. In vitro maturation of human immature oocytes for fertility preservation and research material. Reprod Med Biol. 2017;16:258–67.
Masciangelo R, Bosisio C, Donnez J, Amorim CA, Dolmans M-M. Safety of ovarian tissue transplantation in patients with borderline ovarian tumors. Hum Reprod. 2018;33:212–9.
Soares M, Saussoy P, Maskens M, Reul H, Amorim CA, Donnez J, et al. Eliminating malignant cells from cryopreserved ovarian tissue is possible in leukaemia patients. Br J Haematol. 2017;178:231–9.
Gronier H, Terriou L, Robin G, Wacrenier A, Leroy-Martin B, Lefebvre C, et al. Detection of non-Hodgkin’s lymphoma in ovarian cortex pieces during the process of cryopreservation. J Assist Reprod Genet. 2014;31:1251–5.
Bittinger SE, Nazaretian SP, Gook DA, Parmar C, Harrup RA, Stern CJ. Detection of Hodgkin lymphoma within ovarian tissue. Fertil Steril. 2011;95:803.e3–6.
Stern CJ, Gook D, Hale LG, Agresta F, Oldham J, Rozen G, et al. Delivery of twins following heterotopic grafting of frozen-thawed ovarian tissue. Hum Reprod. 2014;29:1828.
Jensen AK, Kristensen SG, Macklon KT, Jeppesen JV, Fedder J, Ernst E, et al. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Hum Reprod. 2015;30:2838–45.
Ernst EH, Offersen BV, Andersen CY, Ernst E. Legal termination of a pregnancy resulting from transplanted cryopreserved ovarian tissue due to cancer recurrence. J Assist Reprod Genet. 2013;30:975–8.
Rosendahl M, Timmermans Wielenga V, Nedergaard L, Kristensen SG, Ernst E, Rasmussen PE, et al. Cryopreservation of ovarian tissue for fertility preservation: no evidence of malignant cell contamination in ovarian tissue from patients with breast cancer. Fertil Steril. 2011;95:2158–61.
Kyono K, Doshida M, Toya M, Sato Y, Akahira J, Sasano H. Potential indications for ovarian autotransplantation based on the analysis of 5,571 autopsy findings of females under the age of 40 in Japan. Fertil Steril. 2010;93:2429–30.
Luyckx V, Durant JF, Camboni A, Gilliaux S, Amorim CA, Van Langendonckt A, et al. Is transplantation of cryopreserved ovarian tissue from patients with advanced-stage breast cancer safe? A pilot study. J Assist Reprod Genet. 2013;30:1289–99.
Coll L, Parriego M, Boada M, Devesa M, Arroyo G, Rodríguez I, et al. Transition from blastomere to trophectoderm biopsy: comparing two preimplantation genetic testing for aneuploidies strategies. Zygote. 2018;26:191–8.
Liñán A, Lawrenz B, El Khatib I, Bayram A, Arnanz A, Rubio C, et al. Clinical reassessment of human embryo ploidy status between cleavage and blastocyst stage by Next Generation Sequencing. PLoS ONE. 2018;13:e0201652 Yu Y, editor.
Adler A, Lee H-L, McCulloh DH, Ampeloquio E, Clarke-Williams M, Wertz BH, et al. Blastocyst culture selects for euploid embryos: comparison of blastomere and trophectoderm biopsies. Reprod BioMed Online. 2014;28:485–91.
Whitney JB, Balloch K, Anderson RE, Nugent N, Schiewe MC. Day 7 blastocyst euploidy supports routine implementation for cycles using preimplantation genetic testing. JBRA Assist Reprod. 2019;23:45–50.
Zhang XY, Ata B, Son W-Y, Buckett WM, Tan S-L, Ao A. Chromosome abnormality rates in human embryos obtained from in-vitro maturation and IVF treatment cycles. Reprod BioMed Online. 2010;21:552–9.
Spits C, Guzman L, Mertzanidou A, Jacobs K, Ortega-Hrepich C, Gilchrist RB, et al. Chromosome constitution of human embryos generated after in vitro maturation including 3-isobutyl-1-methylxanthine in the oocyte collection medium. Hum Reprod. 2015;30:653–63.
Fesahat F, Kalantar SM, Sheikhha MH, Saeedi H, Montazeri F, Firouzabadi RD, et al. Developmental and cytogenetic assessments of preimplantation embryos derived from in-vivo or in-vitro matured human oocytes. Eur J Med Genet. 2018;61:235–41.
Abir R, Ben-Aharon I, Garor R, Yaniv I, Ash S, Stemmer SM, et al. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Hum Reprod. 2016;31:750–62.
Child TJ, Abdul-Jalil AK, Gulekli B, Lin TS. In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertil Steril. 2001;76:936–42.
Pongsuthirak P, Songveeratham S, Vutyavanich T. Comparison of blastocyst and Sage Media for in vitro maturation of human immature oocytes. Reprod Sci. 2015;22:343–6.
Cha K-Y. Maturation in vitro of immature human oocytes for clinical use. Hum Reprod Update. 1998;4:103–20.
Gruhn JR, Kristensen SG, Andersen CY, Hoffmann ER. In Vitro Maturation and Culture of Human Oocytes. In: Verlhac M-H, Terret M-E, editors. Mouse Oocyte Development. New York: Springer; 2018. p. 23–30. [Internet]. [cited 2020 Jan 15]. Available from: http://link.springer.com/10.1007/978-1-4939-8603-3_3.
Revel A. In vitro maturation and fertilization of oocytes from an intact ovary of a surgically treated patient with endometrial carcinoma: case report. Hum Reprod. 2004;19:1608–11.
Albuz FK, Sasseville M, Lane M, Armstrong DT, Thompson JG, Gilchrist RB. Simulated physiological oocyte maturation (SPOM): a novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum Reprod. 2010;25:2999–3011.
Saenz-de-Juano MD, Ivanova E, Romero S, Lolicato F, Sánchez F, Van Ranst H, et al. DNA methylation and mRNA expression of imprinted genes in blastocysts derived from an improved in vitro maturation method for oocytes from small antral follicles in polycystic ovary syndrome patients. Hum Reprod. 2019;34:1640–9.
Acknowledgments
The authors thank Dr. Zohrab Makiyan for performing the ovariectomy surgery. We also thank Dr. Muminat Ibragimova for providing postoperative care. We are grateful to the personnel of the 1st gynecological department, National Medical Research Center for Obstetrics, Gynecology and Perinatology. We finally want to thank Cerridwen E. Aker for her critical reading of the manuscript.
Author’s roles
AK, TN and GT developed the IVM procedure. AA supervised the IVM procedures. NM, TN and EB were responsible for the diagnostic evaluation and clinical management of the couple. AK and EK were responsible for the laboratory procedures. AE performed preimplantation genetic screening. OB performed testing for mutations associated with breast cancer. MG created the custom panel of the genes. AK wrote the paper. OB and TN edited the paper.
Funding
AK and EB were supported by the Grant of the President of the Russian Federation - for state support of young.
Russian scientists - К-3244.2019.7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed consent
A written informed consent was obtained from the patient.
Ethical approval
This study was approved by the Ethics Committee of our institution (protocol №11 from 13.12.2018).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Table S1
(DOCX 45 kb).
Rights and permissions
About this article
Cite this article
Kirillova, A., Kovalskaya, E., Brovkina, O. et al. Cryopreservation of euploid blastocysts obtained after fertilization of in vitro matured ovarian tissue oocytes: a case report. J Assist Reprod Genet 37, 905–911 (2020). https://doi.org/10.1007/s10815-020-01729-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01729-2