Abstract
Purpose
Balanced carriers of structural rearrangements have an increased risk of unbalanced embryos mainly due to the production of unbalanced gametes during meiosis. Aneuploidy for other chromosomes not involved in the rearrangements has also been described. The purpose of this work is to know if the incidence of unbalanced embryos, interchromosomal effect (ICE) and clinical outcomes differ in carriers of different structural rearrangements.
Methods
Cohort retrospective study including 359 preimplantation genetic testing cycles for structural rearrangements from 304 couples was performed. Comparative genomic hybridisation arrays were used for chromosomal analysis. The results were stratified and compared according to female age and carrier sex. The impact of different cytogenetic features of chromosomal rearrangements was evaluated.
Results
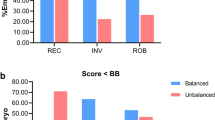
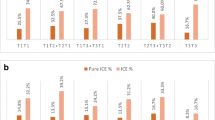
In carriers of translocations, we observed a higher percentage of abnormal embryos from day 3 biopsies compared with day 5/6 biopsies and for reciprocal translocations compared with other rearrangements. We observed a high percentage of embryos with aneuploidies for chromosomes not involved in the rearrangement that could be attributed to total ICE (aneuploid balanced and unbalanced embryos). No significant differences were observed in these percentages between types of rearrangements. Pure ICE (aneuploid balanced embyos) was independent of female age only for Robertsonian translocations, and significantly increased in day 3 biopsies for all types of abnormalities. Furthermore, total ICE for carriers of Robertsonian translocations and biopsy on day 3 was independent of female age too. High ongoing pregnancy rates were observed for all studied groups, with higher pregnancy rate for male carriers.
Conclusion
We observed a higher percentage of abnormal embryos for reciprocal translocations. No significant differences for total ICE was found among the different types of rearrangements, with higher pure ICE only for Robertsonian translocations. There was a sex effect for clinical outcome for carriers of translocations, with higher pregnancy rate for male carriers. The higher incidence of unbalanced and aneuploid embryos should be considered for reproductive counselling in carriers of structural rearrangements.



Similar content being viewed by others
References
Jacobs PA, Melville M, Ratcliffe S, Keay AJ, Syme J. A cytogenetic survey of 11,680 newborn infants. Ann Hum Genet. 1974;37:359–76.
Van Dyke DL, Weiss L, Roberson JR, Babu VR. The frequency and mutation rate of balanced autosomal rearrangements in man estimated from prenatal genetic studies for advanced maternal age. Am J Hum Genet. 1983;35:301–8.
Campana M, Serra A, Neri G. Role of chromosome aberrations in recurrent abortion: a study of 269 balanced translocations. Am J Med Genet. 1986;24:341–56.
Fryns JP, Van Buggenhout G. Structural chromosome rearrangements in couples with recurrent fetal wastage. Eur J Obstet Gynecol Reprod Biol. 1998;81:171–6.
Stern C, Pertile M, Norris H, Hale L, Baker HW. Chromosome translocations in couples with in-vitro fertilization implantation failure. Hum Reprod. 1999;14:2097–101.
Neri G, Serra A, Campana M, Tedeschi B. Reproductive risks for translocation carriers: cytogenetic study and analysis of pregnancy outcome in 58 families. Am J Med Genet. 1983;16:535–61.
Scriven PN, Handyside AH, Ogilvie CM. Chromosome translocations: segregation modes and strategies for preimplantation genetic diagnosis. Prenat Diagn. 1998;18:1437–49.
Anton E, Vidal F, Blanco J. Reciprocal translocations: tracing their meiotic behavior. Genet Med. 2008;10:730–8.
Conn CM, Harper JC, Winston RM, Delhanty JD. Infertile couples with Robertsonian translocations: preimplantation genetic analysis of embryos reveals chaotic cleavage divisions. Hum Genet. 1998;102:117–23.
Fischer J, Colls P, Escudero T, Munne S. Preimplantation genetic diagnosis (PGD) improves pregnancy outcome for translocation carriers with a history of recurrent losses. Fertil Steril. 2010;94:283–9.
Munne S, Sandalinas M, Escudero T, Fung J, Gianaroli L, Cohen J. Outcome of preimplantation genetic diagnosis of translocations. Fertil Steril. 2000;73:1209–18.
Verlinsky Y, Tur-Kaspa I, Cieslak J, Bernal A, Morris R, Taranissi M, et al. Preimplantation testing for chromosomal disorders improves reproductive outcome of poor-prognosis patients. Reprod BioMed Online. 2005;11:219–25.
Munne S, Bahce M, Schimmel T, Sadowy S, Cohen J. Case report: chromatid exchange and predivision of chromatids as other sources of abnormal oocytes detected by preimplantation genetic diagnosis of translocations. Prenat Diagn. 1998;18:1450–8.
Munne S, Morrison L, Fung J, Marquez C, Weier U, Bahce M, et al. Spontaneous abortions are reduced after preconception diagnosis of translocations. J Assist Reprod Genet. 1998;15:290–6.
Verlinsky Y, Evsikov S. Karyotyping of human oocytes by chromosomal analysis of the second polar bodies. Mol Hum Reprod. 1999;5:89–95.
Harper JC, Wilton L, Traeger-Synodinos J, Goossens V, Moutou C, SenGupta SB, et al. The ESHRE PGD Consortium: 10 years of data collection. Hum Reprod Update. 2012;18:234–47.
DeUgarte CM, Li M, Surrey M, Danzer H, Hill D, DeCherney AH. Accuracy of FISH analysis in predicting chromosomal status in patients undergoing preimplantation genetic diagnosis. Fertil Steril. 2008;90:1049–54.
Munne S. Preimplantation genetic diagnosis of numerical and structural chromosome abnormalities. Reprod BioMed Online. 2002;4:183–96.
Velilla E, Escudero T, Munne S. Blastomere fixation techniques and risk of misdiagnosis for preimplantation genetic diagnosis of aneuploidy. Reprod BioMed Online. 2002;4:210–7.
Fiorentino F, Kokkali G, Biricik A, Stavrou D, Ismailoglu B, De Palma R, et al. Polymerase chain reaction-based detection of chromosomal imbalances on embryos: the evolution of preimplantation genetic diagnosis for chromosomal translocations. Fertil Steril. 2010;94:2001-11, 2011.e1-6.
Lukaszuk K, Pukszta S, Ochman K, Cybulska C, Liss J, Pastuszek E, et al. Healthy baby born to a Robertsonian translocation carrier following next-generation sequencing-based preimplantation genetic diagnosis: a case report. AJP Rep. 2015;5:e172–5.
Tan YQ, Tan K, Zhang SP, Gong F, Cheng DH, Xiong B, et al. Single-nucleotide polymorphism microarray-based preimplantation genetic diagnosis is likely to improve the clinical outcome for translocation carriers. Hum Reprod. 2013;28:2581–92.
Treff NR, Tao X, Schillings WJ, Bergh PA, Scott RT Jr, Levy B. Use of single nucleotide polymorphism microarrays to distinguish between balanced and normal chromosomes in embryos from a translocation carrier. Fertil Steril. 2011;96:e58–65.
van Uum CM, Stevens SJ, Dreesen JC, Drusedau M, Smeets HJ, Hollanders-Crombach B, et al. SNP array-based copy number and genotype analyses for preimplantation genetic diagnosis of human unbalanced translocations. Eur J Hum Genet. 2012;20:938–44.
Xie Y, Xu Y, Wang J, Miao B, Zeng Y, Ding C, Gao J, Zhou C. Preliminary analysis of numerical chromosome abnormalities in reciprocal and Robertsonian translocation preimplantation genetic diagnosis cases with 24-chromosomal analysis with an aCGH/SNP microarray. J Assist Reprod Genet. 2018 Jan;35(1):177–186.
Alfarawati S, Fragouli E, Colls P, Wells D. First births after preimplantation genetic diagnosis of structural chromosome abnormalities using comparative genomic hybridization and microarray analysis. Hum Reprod. 2011;26:1560–74.
Fiorentino F, Spizzichino L, Bono S, Biricik A, Kokkali G, Rienzi L, et al. PGD for reciprocal and Robertsonian translocations using array comparative genomic hybridization. Hum Reprod. 2011;26:1925–35.
Li G, Jin H, Xin Z, Su Y, Brezina PR, Benner AT, et al. Increased IVF pregnancy rates after microarray preimplantation genetic diagnosis due to parental translocations. Syst Biol Reprod Med. 2014;60:119–24.
Tobler KJ, Brezina PR, Benner AT, Du L, Xu X, Kearns WG. Two different microarray technologies for preimplantation genetic diagnosis and screening, due to reciprocal translocation imbalances, demonstrate equivalent euploidy and clinical pregnancy rates. J Assist Reprod Genet. 2014;31:843–50.
Bono S, Biricik A, Spizzichino L, Nuccitelli A, Minasi MG, Greco E, et al. Validation of a semiconductor next-generation sequencing-based protocol for preimplantation genetic diagnosis of reciprocal translocations. Prenat Diagn. 2015;35:938–44.
Wang L, Cram DS, Shen J, Wang X, Zhang J, Song Z, et al. Validation of copy number variation sequencing for detecting chromosome imbalances in human preimplantation embryos. Biol Reprod. 2014;91:37.
Lejeune J. Autosomal disorders. Pediatrics. 1963;32:326–37.
Anton E, Vidal F, Blanco J. Interchromosomal effect analyses by sperm FISH: incidence and distribution among reorganization carriers. Syst Biol Reprod Med. 2011;57:268–78.
Anton E, Blanco J, Egozcue J, Vidal F. Sperm FISH studies in seven male carriers of Robertsonian translocation t(13;14)(q10;q10). Hum Reprod. 2004;19:1345–51.
Blanco J, Egozcue J, Clusellas N, Vidal F. FISH on sperm heads allows the analysis of chromosome segregation and interchromosomal effects in carriers of structural rearrangements: results in a translocation carrier, t(5;8)(q33;q13). Cytogenet Cell Genet. 1998;83:275–80.
Douet-Guilbert N, Bris MJ, Amice V, Marchetti C, Delobel B, Amice J, et al. Interchromosomal effect in sperm of males with translocations: report of 6 cases and review of the literature. Int J Androl. 2005;28:372–9.
Escudero T, Lee M, Carrel D, Blanco J, Munne S. Analysis of chromosome abnormalities in sperm and embryos from two 45,XY,t(13;14)(q10;q10) carriers. Prenat Diagn. 2000;20:599–602.
Godo A, Blanco J, Vidal F, Sandalinas M, Garcia-Guixe E, Anton E. Altered segregation pattern and numerical chromosome abnormalities interrelate in spermatozoa from Robertsonian translocation carriers. Reprod BioMed Online. 2015;31:79–88.
Alfarawati S, Fragouli E, Colls P, Wells D. Embryos of robertsonian translocation carriers exhibit a mitotic interchromosomal effect that enhances genetic instability during early development. PLoS Genet. 2012;8:e1003025.
Durban M, Benet J, Boada M, Fernandez E, Calafell JM, Lailla JM, et al. PGD in female carriers of balanced Robertsonian and reciprocal translocations by first polar body analysis. Hum Reprod Update. 2001;7:591–602.
Gutierrez-Mateo C, Gadea L, Benet J, Wells D, Munne S, Navarro J. Aneuploidy 12 in a Robertsonian (13;14) carrier: case report. Hum Reprod. 2005;20:1256–60.
Gianaroli L, Magli MC, Ferraretti AP, Munne S, Balicchia B, Escudero T, et al. Possible interchromosomal effect in embryos generated by gametes from translocation carriers. Hum Reprod. 2002;17:3201–7.
Tulay P, Gultomruk M, Findikli N, Yagmur E, Bahceci M. Is the interchromosomal effect present in embryos derived from Robertsonian and reciprocal translocation carriers particularly focusing on chromosome 10 rearrangements? Zygote. 2015;23:908–15.
Rubio C, Mercader A, Alama P, Lizan C, Rodrigo L, Labarta E, et al. Prospective cohort study in high responder oocyte donors using two hormonal stimulation protocols: impact on embryo aneuploidy and development. Hum Reprod. 2010;25:2290–7.
Cobo A, Bellver J, Domingo J, Perez S, Crespo J, Pellicer A, et al. New options in assisted reproduction technology: the Cryotop method of oocyte vitrification. Reprod BioMed Online. 2008;17:68–72.
Beyer CE, Willats E. Natural selection between day 3 and day 5/6 PGD embryos in couples with reciprocal or Robertsonian translocations. J Assist Reprod Genet. 2017 Nov;34(11):1483–1492.
Amir H, Barbash-Hazan S, Kalma Y, Frumkin T, Malcov M, Samara N, et al. Time-lapse imaging reveals delayed development of embryos carrying unbalanced chromosomal translocations. J Assist Reprod Genet. 2019;36:315–24.
Rodrigo L, Mateu E, Mercader A, Cobo AC, Peinado V, Milan M, et al. New tools for embryo selection: comprehensive chromosome screening by array comparative genomic hybridization. Biomed Res Int. 2014;2014:517125.
Otani T, Roche M, Mizuike M, Colls P, Escudero T, Munne S. Preimplantation genetic diagnosis significantly improves the pregnancy outcome of translocation carriers with a history of recurrent miscarriage and unsuccessful pregnancies. Reprod BioMed Online. 2006;13:869–74.
Zhang S, Lei C, Wu J, Sun H, Zhou J, Zhu S, et al. Analysis of segregation patterns of quadrivalent structures and the effect on genome stability during meiosis in reciprocal translocation carriers. Hum Reprod. 2018;33:757–67.
Jalbert P, Sele B, Jalbert H. Reciprocal translocations: a way to predict the mode of imbalanced segregation by pachytene-diagram drawing. Hum Genet. 1980;55:209–22.
Chelli MH, Ferfouri F, Boitrelle F, Albert M, Molina-Gomes D, Selva J, et al. High-magnification sperm selection does not decrease the aneuploidy rate in patients who are heterozygous for reciprocal translocations. J Assist Reprod Genet. 2013;30:525–30.
Estop AM, Cieply K, Munne S, Surti U, Wakim A, Feingold E. Is there an interchromosomal effect in reciprocal translocation carriers? Sperm FISH studies. Hum Genet. 2000;106:517–24.
Kirkpatrick G, Ren H, Liehr T, Chow V, Ma S. Meiotic and sperm aneuploidy studies in three carriers of Robertsonian translocations and small supernumerary marker chromosomes. Fertil Steril. 2015;103:1162-9.e7.
Luciani JM, Guichaoua MR, Mattei A, Morazzani MR. Pachytene analysis of a man with a 13q;14q translocation and infertility. Behavior of the trivalent and nonrandom association with the sex vesicle. Cytogenet Cell Genet. 1984;38:14–22.
Navarro J, Vidal F, Benet J, Templado C, Marina S, Egozcue J. XY-trivalent association and synaptic anomalies in a male carrier of a Robertsonian t(13;14) translocation. Hum Reprod. 1991;6:376–81.
Rogenhofer N, Durl S, Ochsenkuhn R, Neusser M, Aichinger E, Thaler CJ, et al. Case report: elevated sperm aneuploidy levels in an infertile Robertsonian translocation t(21;21) carrier with possible interchromosomal effect. J Assist Reprod Genet. 2012;29:343–6.
Pujol A, Durban M, Benet J, Boiso I, Calafell JM, Egozcue J, et al. Multiple aneuploidies in the oocytes of balanced translocation carriers: a preimplantation genetic diagnosis study using first polar body. Reproduction. 2003;126:701–11.
Ghevaria H, SenGupta S, Shmitova N, Serhal P, Delhanty J. The origin and significance of additional aneuploidy events in couples undergoing preimplantation genetic diagnosis for translocations by array comparative genomic hybridization. Reprod BioMed Online. 2016;32:178–89.
Moosani N, Pattinson HA, Carter MD, Cox DM, Rademaker AW, Martin RH. Chromosomal analysis of sperm from men with idiopathic infertility using sperm karyotyping and fluorescence in situ hybridization. Fertil Steril. 1995;64:811–7.
Pang MG, Hoegerman SF, Cuticchia AJ, Moon SY, Doncel GF, Acosta AA, et al. Detection of aneuploidy for chromosomes 4, 6, 7, 8, 9, 10, 11, 12, 13, 17, 18, 21, X and Y by fluorescence in-situ hybridization in spermatozoa from nine patients with oligoasthenoteratozoospermia undergoing intracytoplasmic sperm injection. Hum Reprod. 1999;14:1266–73.
Rubio C, Bellver J, Rodrigo L, Castillon G, Guillen A, Vidal C, et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril. 2017;107:1122–9.
Rubio C, Gil-Salom M, Simon C, Vidal F, Rodrigo L, Minguez Y, et al. Incidence of sperm chromosomal abnormalities in a risk population: relationship with sperm quality and ICSI outcome. Hum Reprod. 2001;16:2084–92.
Xie Y, Xu Y, Wang J, Miao B, Zeng Y, Ding C, et al. Preliminary analysis of numerical chromosome abnormalities in reciprocal and Robertsonian translocation preimplantation genetic diagnosis cases with 24-chromosomal analysis with an aCGH/SNP microarray. J Assist Reprod Genet. 2018;35:177–86.
Iews M, Tan J, Taskin O, Alfaraj S, AbdelHafez FF, Abdellah AH, et al. Does preimplantation genetic diagnosis improve reproductive outcome in couples with recurrent pregnancy loss owing to structural chromosomal rearrangement? A systematic review. Reprod BioMed Online. 2018;36:677–85.
Ikuma S, Sato T, Sugiura-Ogasawara M, Nagayoshi M, Tanaka A, Takeda S. Preimplantation genetic diagnosis and natural conception: a comparison of live birth rates in patients with recurrent pregnancy loss associated with translocation. PLoS One. 2015;10:e0129958.
Keymolen K, Staessen C, Verpoest W, Liebaers I, Bonduelle M. Preimplantation genetic diagnosis in female and male carriers of reciprocal translocations: clinical outcome until delivery of 312 cycles. Eur J Hum Genet. 2012;20:376–80.
Maithripala S, Durland U, Havelock J, Kashyap S, Hitkari J, Tan J, et al. Prevalence and treatment choices for couples with recurrent pregnancy loss due to structural chromosomal anomalies. J Obstet Gynaecol Can. 2018;40:655–62.
Kato K, Aoyama N, Kawasaki N, Hayashi H, Xiaohui T, Abe T, et al. Reproductive outcomes following preimplantation genetic diagnosis using fluorescence in situ hybridization for 52 translocation carrier couples with a history of recurrent pregnancy loss. J Hum Genet. 2016;61:687–92.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the institutional review and ethical board at the Instituto Valenciano de Infertilidad (1503-IGX-020-EM).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary materials
ESM 1
Supplementary Table I. Categorization of embryos stratified by maternal age, Supplementary Table II.Categorization of embryos according to sex of the carrier of the rearrangement, Supplementary Table III. Clinical outcome according to maternal age and structural rearrangement, Supplementary Table IV. Clinical outcome according to sex of the carrier of the rearrangement (DOCX 53 kb)
Rights and permissions
About this article
Cite this article
Mateu-Brull, E., Rodrigo, L., Peinado, V. et al. Interchromosomal effect in carriers of translocations and inversions assessed by preimplantation genetic testing for structural rearrangements (PGT-SR). J Assist Reprod Genet 36, 2547–2555 (2019). https://doi.org/10.1007/s10815-019-01593-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01593-9