Abstract
Purpose
In this study, we tested the hypothesis that, in PGT-A cycles, decreased semen quality is associated with increased rates of mosaic blastocysts.
Methods
In a retrospective analysis, three hundred and forty PGT-A cycles are divided into study groups according to semen quality. Cycles were initially divided into two groups, discerning couples with absence of male factor of infertility (non-male factor: NMF; N = 146 cycles) from couples with a male factor of infertility (MF; N = 173 cycles). Couples with severe male factor (SMF) infertility (n = 22) were assessed separately. Embryos were cultured to the blastocyst stage and chromosomally assessed by array comparative genomic hybridization (aCGH). The study did not involve specific interventions.
Results
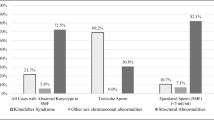
The reproductive outcome of MF and NMF groups did not indicate statistically significant differences. However, while no differences were found between MF and NMF groups in terms of euploid or aneuploid blastocysts rates, a significantly higher rate of mosaic blastocysts was observed in the MF group (3.6% vs. 0.5%, respectively; P = 0.03). A similar pattern of results was observed in the SMF group when compared with those of the other PGT-A cycles taken together (no SMF). In particular, a significantly higher rate of mosaic blastocysts was observed in the SMF group (7.7% and 1.8%, respectively; P = 0.008).
Conclusions
The study outcome strongly suggests that compromised semen quality is associated with increased rates of mosaic blastocysts analysed in PGT-A cycles. Sperm assessment appears therefore as an important factor in the determination of embryo development and for a more precise prognostic assessment of PGT-A cases.

Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Griffin DK, Ogur C. Chromosomal analysis in IVF: just how useful is it? Reproduction. 2018;156:F29–50.
Fragouli E, Munne S, Wells D. The cytogenetic constitution of human blastocysts: insights from comprehensive chromosome screening strategies. Hum Reprod Update. 2019;25:15–33.
Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–91.
Capalbo A, Hoffmann ER, Cimadomo D, Ubaldi FM, Rienzi L. Human female meiosis revised: new insights into the mechanisms of chromosome segregation and aneuploidies from advanced genomics and time-lapse imaging. Hum Reprod Update. 2017;23:706–22.
McCoy RC. Mosaicism in preimplantation human embryos: when chromosomal abnormalities are the norm. Trends Genet. 2017;33:448–63.
Munné S, Wells D. Detection of mosaicism at blastocyst stage with the use of high-resolution next-generation sequencing. Fertil Steril. 2017;107:1085–91.
Munné S. Origins of mosaicism and criteria for the transfer of mosaic embryos. Reprod BioMed Online. 2018;36:369–70.
Sekhon L, Feuerstein J, Nazem TG, Briton-Jones C, Lee JA, Grunfeld L, et al. The incidence of mosaicism is not associated with advanced maternal age or diminished ovarian reserve. Fertil Steril [Internet. 2017;108(3):e217.
Tarozzi N, Nadalini M, Bizzaro D, Serrao L, Fava L, Scaravelli G, et al. Sperm-hyaluronan-binding assay: clinical value in conventional IVF under Italian law. Reprod BioMed Online. 2009;19(Suppl 3):35–43.
Borini A, Bonu MA, Coticchio G, Bianchi V, Cattoli M, Flamigni C. Pregnancies and births after oocyte cryopreservation. Fertil Steril. 2004;82:601–5.
Borini A, Bafaro MG, Bianchi L, Violini F, Bonu MA, Flamigni C. Oocyte donation programme: results obtained with intracytoplasmic sperm injection in cases of severe male factor infertility or previous failed fertilisation. Hum Reprod. 1996;11:548–50.
Lagalla C, Tarozzi N, Sciajno R, Wells D, Di Santo M, Nadalini M, et al. Embryos with morphokinetic abnormalities may develop into euploid blastocysts. Reprod BioMed Online. 2017;34:137–46.
Fragouli E, Alfarawati S, Spath K, Wells D. Morphological and cytogenetic assessment of cleavage and blastocyst stage embryos. Mol Hum Reprod. 2014;20:117–26.
Fragouli E, Alfarawati S, Spath K, Babariya D, Tarozzi N, Borini A, et al. Analysis of implantation and ongoing pregnancy rates following the transfer of mosaic diploid-aneuploid blastocysts. Hum Genet. 2017;136:805–19.
Cobo A, Bellver J, Domingo J, Pérez S, Crespo J, Pellicer A, et al. New options in assisted reproduction technology: the Cryotop method of oocyte vitrification. Reprod BioMed Online. 2008;17:68–72.
Zacà C, Bazzocchi A, Pennetta F, Bonu MA, Coticchio G, Borini A. Cumulative live birth rate in freeze-all cycles is comparable to that of a conventional embryo transfer policy at the cleavage stage but superior at the blastocyst stage. Fertil Steril. 2018;110:703–9.
Webster A, Schuh M. Mechanisms of aneuploidy in human eggs. Trends Cell Biol. 2017;27:55–68.
Taylor TH, Gitlin SA, Patrick JL, Crain JL, Wilson JM, Griffin DK. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum Reprod Update. 2014;20:571–81.
Mantikou E, Wong KM, Repping S, Mastenbroek S. Molecular origin of mitotic aneuploidies in preimplantation embryos. Biochim Biophys Acta. 2012;1822:1921–30.
Bean CJ, Hunt PA, Millie EA, Hassold TJ. Analysis of a malsegregating mouse Y chromosome: evidence that the earliest cleavage divisions of the mammalian embryo are non-disjunction-prone. Hum Mol Genet. 2001;10:963–72.
Coonen E, Derhaag JG, Dumoulin JC, van WLC, Bras M, Janssen M, et al. Anaphase lagging mainly explains chromosomal mosaicism in human preimplantation embryos. Hum Reprod. 2004;19:316–24.
Capalbo A, Bono S, Spizzichino L, Biricik A, Baldi M, Colamaria S, et al. Sequential comprehensive chromosome analysis on polar bodies, blastomeres and trophoblast: insights into female meiotic errors and chromosomal segregation in the preimplantation window of embryo development. Hum Reprod. 2013;28:509–18.
Katz-Jaffe MG, Trounson AO, Cram DS. Chromosome 21 mosaic human preimplantation embryos predominantly arise from diploid conceptions. Fertil Steril. 2005;84:634–43.
Baart EB, Martini E, Eijkemans MJ, Van OD, Beckers NG, Verhoeff A, et al. Milder ovarian stimulation for in-vitro fertilisation reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod. 2007;22:980–8.
Munne S, Magli C, Adler A, Wright G, de BK, Mortimer D, et al. Treatment-related chromosome abnormalities in human embryos. Hum Reprod. 1997;12:780–4.
Verpoest W, Fauser BC, Papanikolaou E, Staessen C, Van LL, Donoso P, et al. Chromosomal aneuploidy in embryos conceived with unstimulated cycle IVF. Hum Reprod. 2008;23:2369–71.
Bean CJ, Hassold TJ, Judis L, Hunt PA. Fertilisation in vitro increases non-disjunction during early cleavage divisions in a mouse model system. Hum Reprod. 2002;17:2362–7.
Baumann C, Viveiros MM, De LFR. Loss of maternal ATRX results in centromere instability and aneuploidy in the mammalian oocyte and pre-implantation embryo. PLoS Genet. 2010;6:e1001137.
Liu L, Blasco MA, Keefe DL. Requirement of functional telomeres for metaphase chromosome alignments and integrity of meiotic spindles. EMBO Rep. 2002;3:230–4.
Wells D, Bermudez MG, Steuerwald N, Thornhill AR, Walker DL, Malter H, et al. Expression of genes regulating chromosome segregation, the cell cycle and apoptosis during human preimplantation development. Hum Reprod. 2005;20:1339–48.
Zheng P, Dean J. Role of Filia, a maternal effect gene, in maintaining euploidy during cleavage-stage mouse embryogenesis. Proc Natl Acad Sci U S A. 2009;106:7473–8.
Brooker AS, Berkowitz KM. The roles of cohesins in mitosis, meiosis, and human health and disease. Methods Mol Biol. 2014;1170:229–66.
Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, et al. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol. 2010;20:1511–21.
Tsutsumi M, Fujiwara R, Nishizawa H, Ito M, Kogo H, Inagaki H, et al. Age-related decrease of meiotic cohesins in human oocytes. PLoS One. 2014;9:e96710.
Munne S. Chromosome abnormalities in human embryos. Hum Reprod Update [Internet. 1998;4(6):842–55. https://doi.org/10.1093/humupd/4.6.842.
Munné S, Cohen J, Sable D. Preimplantation genetic diagnosis for advanced maternal age and other indications. Fertil Steril. 2002;78:234–6.
Palermo G, Munné S, Cohen J. The human zygote inherits its mitotic potential from the male gamete. Hum Reprod. 1994;9:1220–5.
Sathananthan AH, Kola I, Osborne J, Trounson A, Ng SC, Bongso A, et al. Centrioles in the beginning of human development. Proc Natl Acad Sci U S A. 1991;88:4806–10.
Terada Y, Nakamura S, Morita J, Tachibana M, Morito Y, Ito K, et al. Use of mammalian eggs for assessment of human sperm function: molecular and cellular analyses of fertilisation by intracytoplasmic sperm injection. Am J Reprod Immunol. 2004;51:290–3.
Yoshimoto-Kakoi T, Terada Y, Tachibana M, Murakami T, Yaegashi N, Okamura K. Assessing centrosomal function of infertile males using heterologous ICSI. Syst Biol Reprod Med. 2008;54:135–42.
Silber S, Escudero T, Lenahan K, Abdelhadi I, Kilani Z, Munné S. Chromosomal abnormalities in embryos derived from testicular sperm extraction. Fertil Steril. 2003;79:30–8.
Magli MC, Gianaroli L, Ferraretti AP, Gordts S, Fredericks V, Crippa A. Paternal contribution to aneuploidy in preimplantation embryos. Reprod BioMed Online. 2009;18:536–42.
Borges E Jr, Zanetti BF, Setti AS, Braga DPAF, Provenza RR, Iaconelli A Jr. Sperm DNA fragmentation is correlated with poor embryo development, lower implantation rate, and higher miscarriage rate in reproductive cycles of non-male factor infertility. Fertil Steril. 2019;112:483–90. https://doi.org/10.1016/j.fertnstert.2019.04.029.
Templado C, Uroz L, Estop A. New insights on the origin and relevance of aneuploidy in human spermatozoa. Mol Hum Reprod. 2013;19:634–43.
Mazzilli R, Cimadomo D, Vaiarelli A, Capalbo A, Dovere L, Alviggi E, et al. Effect of the male factor on the clinical outcome of intracytoplasmic sperm injection combined with preimplantation aneuploidy testing: observational longitudinal cohort study of 1,219 consecutive cycles. Fertil Steril. 2017;108:961–72.e3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 31 kb)
Rights and permissions
About this article
Cite this article
Tarozzi, N., Nadalini, M., Lagalla, C. et al. Male factor infertility impacts the rate of mosaic blastocysts in cycles of preimplantation genetic testing for aneuploidy. J Assist Reprod Genet 36, 2047–2055 (2019). https://doi.org/10.1007/s10815-019-01584-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01584-w