Abstract
Purpose
Implantation of the mammalian embryo in the uterus is preceded by escape from the zona pellucida. In some species, hatching from the zona occurs preferentially from one or the other poles of the embryo. The situation for the bovine embryo, in which hatching precedes attachment to the uterus by more than a week, is unclear. The purpose was to describe whether hatching of the bovine embryo from the zona pellucida occurs preferentially from the embryonic or abembryonic pole.
Methods
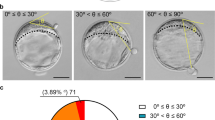
Bovine blastocysts undergoing hatching were examined by light microscopy (n = 84) and epifluorescence imaging using antibodies for markers of epiblast, hypoblast, and trophectoderm (TE) (n = 26). The location of hatching was classified as being at the embryonic pole, if hatching occurred ipsilateral to the inner cell mass (ICM), or abembryonic, if hatching occurred contralateral to the ICM.
Results
A total of 55% of blastocysts exited the zona pellucida through an opening at the embryonic pole. In these cases, 68% of the cells emerging through the zona pellucida were derived from the ICM. The remainder of blastocysts hatched from an opening either contralateral or to the side of the ICM. In these cases, 87% of hatched cells were TE.
Conclusion
For the bovine embryo, there is nearly equal probability of hatching from the embryonic or abembryonic poles. Given that the surface area of the zona pellucida in contact with the TE overlying the ICM is less than for the remainder of the blastocyst, there is some preference for hatching through the embryonic pole. Thus, the bovine embryo is distinct from the mouse and human, where hatching occurs preferentially at the abembryonic pole.



Similar content being viewed by others
References
Carney S-KK, Das S, Blake D, Farquhar C, Seif MWMW, Nelson L. Assisted hatching on assisted conception (in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI)). Cochrane Database Syst Rev. 2012;12:CD001894.
Taniyama A, Watanabe Y, Nishino Y, Inoue T. Assisted hatching of poor-quality bovine embryos increases pregnancy. J Reprod Dev. 2011;57:543–6.
Cole RJ. Cinemicrographic observations on the trophoblast and zona pellucida of the mouse blastocyst. J Embryol Exp Morphol. 1967;17:481–90.
Massip A, Mulnard J. Time-lapse cinematographic analysis of hatching of normal and frozen-thawed cow blastocysts. J Reprod Fertil. 1980;58:475–8.
Massip A, Mulnard J, Vanderzwalmen P, Hanzen C, Ectors F. The behaviour of cow blastocyst in vitro: cinematographic and morphometric analysis. J Anat. 1982;134:399–405.
Sawada H, Yamazaki K, Hoshi M. Trypsin-like hatching protease from mouse embryos: evidence for the presence in culture medium and its enzymatic properties. J Exp Zool. 1990;254:83–7.
Mishra A, Seshagiri PB. Evidence for the involvement of a species-specific embryonic protease in zona escape of hamster blastocysts. Mol Hum Reprod. 2000;6:1005–12.
Berg DA, Menino AR Jr. Bovine embryos produce urokinase-type plasmogen activator. Mol Reprod Dev. 1992;31:14–9.
Gonzales DS, Jones JM, Pinyopummintr T, Carnevale EM, Ginther OJ, Shapiro SS, Bavister BD. Trophectoderm projections: a potential means for locomotion, attachment and implantation of bovine, equine and human blastocysts. Hum Reprod. 1996;11:2739–45.
Seshagiri PB, Sen Roy S, Sireesha G, Rao RP. Cellular and molecular regulation of mammalian blastocyst hatching. J Reprod Immunol. 2009;83:79–84.
O’Sullivan CM, Rancourt SL, Liu SY, Rancourt DE. A novel murine tryptase involved in blastocyst hatching and outgrowth. Reproduction. 2001;122:61–71.
Perona RM, Wassarman PM. Mouse blastocysts hatch in vitro by using a trypsin-like proteinase associated with cells of mural trophectoderm. Dev Biol. 1986;114:42–52.
Sireesha GV, Mason RW, Hassanein M, Tonack S, Navarrete Santos A, Fischer B, Seshagiri PB. Role of cathepsins in blastocyst hatching in the golden hamster. Mol Hum Reprod. 2008;14:337–46.
Coates AA, Menino AR. Effects of blastocoelic expansion and plasminogen activator activity on hatching and zona pellucida solubility in bovine embryos in vitro. J Anim Sci. 1994;72:2936–42.
Sathananthan H, Menezes J, Gunasheela S. Mechanics of human blastocyst hatching in vitro. Reprod BioMed Online. 2003;7:228–34.
Spee GF. Beitrag zur entwickelungsgeschichte der fruheren stadien des meerschweinchens bis zur vollendung der keimblase. Arch Anat Physiol. 1883;7:44–60.
Betteridge KJ, Flechon JE. The anatomy and physiology of pre- attachement bovine embryos. Theriogenology. 1988;29:155–87.
King GJ, Atkinson BA, Robertson HA. Development of the intercaruncular areas during early gestation and establishment of the bovine placenta. J Reprod Fert. 1981;61:469–74.
Niimura S, Ogata T, Okimura A, Sato T, Uchiyama Y, Seta T, Nakagawa H, Nakagawa K, Tamura Y. Time-lapse videomicrographic observations of blastocyst hatching in cattle. J Reprod Dev. 2010;56:649–54.
Strumpf D, Mao CA, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, Rossant J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–102.
Chen L, Wang D, Wu Z, Ma L, Daley GQ. Molecular basis of the first cell fate determination in mouse embryogenesis. Cell Res. 2010;20:982–93.
Denicol AC, Block J, Kelley DE, Pohler KG, Dobbs KB, Mortensen CJ, Ortega MS, Hansen PJ. The WNT signaling antagonist Dickkopf-1 directs lineage commitment and promotes survival of the preimplantation embryo. FASEB J. 2014:1–12.
Ortega MS, Wohlgemuth S, Tribulo P, Siqueira LGB, Null DJ, Cole JB, Da Silva M V., Hansen PJ. A single nucleotide polymorphism in COQ9 affects mitochondrial and ovarian function and fertility in Holstein cows. Biol Reprod 2017; 0:1–12.
Kannampuzha Francis J, Tribulo P, Hansen PJ. Actions of activin A, connective tissue growth factor, hepatocyte growth factor and teratocarcinoma—derived growth factor 1 on the development of the bovine preimplantation embryo. Reprod Fertil Dev 2017:1–13 (in press).
Nagatomo H, Kagawa S, Kishi Y, Takuma T, Sada A, Yamanaka K-I, Abe Y, Wada Y, Takahashi M, Kono T, Kawahara M. Transcriptional wiring for establishing cell lineage specification at the blastocyst stage in cattle. Biol Reprod. 2013;88:158.
Lonergan P, Forde N. Maternal-embryo interaction leading up to the initiation of implantation of pregnancy in cattle. Animal. 2014;8(Suppl 1):64–9.
Gómez E, Muñoz M. Multiple-embryo transfer for studying very early maternal-embryo interactions in cattle. Reproduction. 2015;150:R35–43.
Gonzales DS, Bavister BD. Zona pellucida escape by hamster blastocysts in vitro is delayed and morphologically different compared with zona escape in vivo. Biol Reprod. 1995;52:470–80.
Acknowledgements
Verónica Negrón-Pérez was supported by a McKnight Doctoral Fellowship from the Florida Education Fund, Inc. The authors thank owners and employees of Central Beef Packing Co. (Center Hill, FL), Adena Meat Products L.P. (Fort McCoy, FL), and Florida Beef Inc. (Zolfo Springs, FL) for providing ovaries; William Rembert and Eddie Cummings for ovary collection; and Doug Smith and the McKnight Brain Institute Cell Tissue and Analysis Core of the University of Florida for assistance with imaging and processing of confocal microscopy figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by USDA-NIFA AFRI Grant No. 2011-67015-30688 and the L.E. “Red” Larson Endowment.
Conflict of interest
The authors declare that they have no conflict of interest. This article does not contain any studies with human participants or animals performed by any of the authors.
Grant support
USDA-NIFA AFRI Grant No. 2011-67015-30688.
Rights and permissions
About this article
Cite this article
Negrón-Pérez, V.M., Hansen, P.J. The bovine embryo hatches from the zona pellucida through either the embryonic or abembryonic pole. J Assist Reprod Genet 34, 725–731 (2017). https://doi.org/10.1007/s10815-017-0933-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-0933-3