Abstract
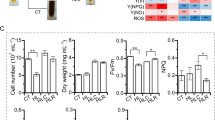
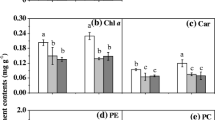
Long-term acclimation to monochromatic lights emerges in seaweeds. In this study, analysis based on physiological characteristics and transcriptome sequencing were employed to investigate the long-term acclimation of thalli of rhodophyte Pyropia haitanensis grown under white light (WL) and monochromatic blue light (BL), green light (GL), and red light (RL). The net photosynthesis of synchronically cultured thalli was highest under WL, while the net photosynthesis was significantly higher under GL and RL compared with BL, indicating the low utilization efficiency of BL and relatively high ones of GL and RL. Compared with WL, the PE/Chl. a ratio was significantly higher in BL-acclimated thalli and lower in both GL- and RL-acclimated thalli. The C/N ratio was decreased in BL-acclimated thalli and drastically increased in GL- and RL-acclimated thalli. Only a small amount of starch grains were observed in WL-acclimated thallus cells, and no starch grain was found in BL-acclimated thallus cells, whereas numerous starch grains were found in GL- and RL-acclimated thallus cells. These findings implied roles of pigments and allocation of carbon between carbohydrates and phycobiliproteins during monochromatic light acclimation. Transcriptome analysis showed that DEGs involving protein synthesis were mainly upregulated by BL, whereas DEGs involving energy-yielding carbohydrate catabolism were mainly downregulated by GL and RL. Besides, genes encoding light-harvesting complex, ferredoxin-NADP reductase, and key enzymes of carbon and nitrogen assimilation were upregulated by BL, yet downregulated by GL and RL. Those results indicated that P. haitanensis thalli could compensate for the low utilization efficiency of BL via the increase in phycoerythrin accumulation through upregulating enzymes acting on carbon and nitrogen assimilation, consequently activating downstream of protein synthesis. Mechanisms of photoacclimation under GL and RL might include the reduction in light energy absorption and photosynthetic electron transfer chain activity via downregulating genes encoding nitrogen assimilating enzymes, light-harvesting complexes and ferredoxin-NADP reductases, and the attenuation in accumulation and catabolism of photosynthates to avoid excessive energy-yielding that might cause a burden on metabolism.







Similar content being viewed by others
Data availability
The raw data generated in this study were deposited in the NCBI database (GenBank accession number: SRP222681).
References
Aguilera J, Gordillo L, Karsten U, Figueroa LF, Niell FX (2000) Light quality effect on photosynthesis and efficiency of carbon assimilation in the red alga Porphyra leucosticta. J Plant Physiol 157:86–92
Banerjee A, Roychoudhury A (2016) Plant responses to light stress: oxidative damages, photoprotection, and role of phytohormones. In: Ahammed GJ, Yu JQ (eds) Plant hormones under challenging environmental factors. Springer, Dordrecht, pp 181–213
Beer S, Levy I (1983) Effects of photon fluence rate and light spectrum on growth, photosynthesis and pigment compositions in Gracilaria sp. J Phycol 19:516–522
Bird TK, Dawes CJ, Romeo JT (1981) Light quality effects on carbon metabolism and allocation in Gracilaria verrucosa. Mar Biol 64:219–223
Calabrese G, Felicini GP (1973) Research on red algal pigments. 5. The effect of white and green light on the rate of photosynthesis and its relationship to pigment components in Gracilaria compressa (C. Ag.) Grev. (Rhodophyceae, Gigartinales). Phycologia 12:195–199
Champigny ML (1995) Integration of photosynthetic carbon and nitrogen metabolism in higher plants. Photosynth Res 46:117–127
Demmig-Adams B, Adams W (2000) Harvesting sunlight safely. Nature 403:371–373
Diakoff S, Scheibe J (1973) Action spectra for chromatic adaptation in Tolypothrix tenuis. Plant Physiol 51:382–385
Dring MJ (1981) Chromatic adaptation of photosynthesis in benthic marine algae: An examination of its ecological significance using a theoretical model. Limnol Oceanogr 26:271–284
Dring MJ (1988) Photocontrol in development in algae. Annu Rev Plant Physiol Plant Mol Biol 39:199–207
Dring MJ, Lüning K (1985) Emerson enhancement effect and quantum yield of photosynthesis for marine macroalgae in simulated underwater light fields. Mar Biol 87:109–117
Engelmann TW (1902) Ueber experimentelle Erzeugung zweckmaessiger Aenderungen der Faerbung pflaenzlicher Chromophylle durch farbiges Licht. Bericht ueber Versuche von Dr. N. Gaidukov. Archiv fuer Anatomie und Physiologie. Physiolog Abteilung, pp 333-335
Fernández E, Llamas Á, Galván A (2009) Nitrogen assimilation and its regulation. In: Harris EH, Stern DB, Witman GB (eds) The Chlamydomonas sourcebook, Vol 2, 2nd edn. Academic Press, London pp 69–113
Gao HF (1993) The variation in the contents of phycobiliproteins from Porphyra haitanensis collected in different growing stages. Oceanol Limnol Sin 24:645–648 (in Chinese with English abstract)
Gao HF, Cao WD, Ji MH (1993) Studies on chemical properties and components of phycobiliproteins from Porphyra haitanensis. Oceanol Limnol Sin 24:350–355 (in Chinese with English abstract)
Glover HE, Keller MD, Guillard RRL (1986) Light quality and oceanic ultraphytoplankters. Nature 319:142–143
Glover HE, Keller MD, Spinrad RW (1987) The effects of light quality and intensity on photosynthesis and growth of marine eukaryotic and prokaryotic phytoplankton clones. J Exp Mar Biol Ecol 105:137–159
Hannach G (1989) Spectral light absorption by intact blades of Porphyra abbottae (Rhodophyta): Effects of environmental factors in culture. J Phycol 25:522–529
Haury JF, Bogorad L (1977) Action spectra for phycobiliprotein synthesis in a chromatically adapting cyanophyte, Fremyella diplosiphon. Plant Physiol 60:835–839
Haxo FT, Blinks LR (1950) Photosynthetic action spectra of marine algae. J Gen Physiol 33:389–422
Kamiya A, Miyachi S (1984) Blue-green and green light adaptations on photosynthetic activity in some algae collected from subsurface chlorophyll layer in the western Pacific Ocean. In: Senger H (Ed) Blue light effects in biological systems. Springer, Berlin pp517-528
Kirk JTO (1994) Light and photosynthesis in aquatic ecosystems. Cambridge University Press, Cambridge
Lapointe BE (1981) The effects of light and nitrogen on growth, pigment content, and biochemical composition of Gracilaria foliifera v. angustissima (Gigartinales, Rhodophyta). J Phycol 17:90–95
Larkum AWD, Weyrauch SK (1977) Photosynthetic action spectra and light harvesting in Griffithsia monilis. Photochem Photobiol 25:65–72
Leukart P, Lüning K (1994) Minimum spectral light requirements and maximum light levels for long-term germling growth of several red algae from different water depths and a green alga. Eur J Phycol 29:103–112
López-Figueroa F (1992) Diurnal variation in pigment content in Porphyra laciniata and Chondrus crispus and its relation to the diurnal changes of underwater light quality and quantity. Mar Biol 13:285–305
López-Figueroa F, Aguilera J, Niell FX (1995) Red and blue light regulation of growth and photosynthetic metabolism in Porphyra umbilicalis (Bangiales, Rhodophyta). Eur J Phycol 30:11–18
López-Figueroa F, Niell FX (1990) Effects of light quality on chlorophyll and biliprotein accumulation in seaweeds. Mar Biol 104:321–327
Lüning K, Dring MJ (1985) Action spectra and spectral quantum yield of photosynthesis in marine macroalgae with thin and thick thalli. Mar Biol 87:119–129
Mulo P (2011) Chloroplast-targeted ferredoxin-NADP+ oxidoreductase (FNR): Structure, function and location. Biochim Biophys Acta Bioenergetics 1807:927–934
Ramus J (1983) A physiological test of the theory of complementary chromatic adaptation. II. Brown, green and red seaweeds. J Phycol 19:173–178
Ramus J, Beale SI, Mauzerall D (1976) Correlation of changes in pigment content with photosynthetic capacity of seaweeds in a function of water depth. Mar Biol 37:231–238
Ramus J, van der Meer JP (1983) A physiological test of the theory of complementary chromatic adaptation. I. Color mutants of a red seaweed. J Phycol 19:86–91
Sanfilippo JE, Garczarek L, Partensky F, Kehoe DM (2019) Chromatic acclimation in cyanobacteria: a diverse and widespread process for optimizing photosynthesis. Annu Rev Microbiol 73:407–433
Taiz L, Zeiger E, Song CP (2015) Plant Physiology. Science Press, Beijing
Talarico L, Maranzana G (2000) Light and adaptative responses in red macroalgae: an overview. J Photochem Photobiol B 56:1–11
Tsekos I, Niell FX, Aguilera JA, López-Figuero F, Delivopoulos SG (2002) Ultrastructure of the vegetative gametophytic cells of Porphyra leucosticte (Rhodophyta) grown in red, blue and green light. Phycol Res 50:251–264
Vogelmann TC, Scheibe J (1978) Action spectrum for chromatic adaptation in the blue-green alga Fremyella diplosiphon. Planta 143:233–239
Wang SJ, Zhang XP, Xu ZD, Sun YL (1986) A study on the cultivation of the vegetative cells and protoplasts of P. haitanensis I. Oceanol Limnol Sinica 17:217–221 (in Chinese with English abstract)
Webber AN, Nie GY, Long SP (1994) Acclimation of photosynthetic proteins to rising atmospheric CO2. Photosynth Res 39:413–425
Wu XJ, Niu JF, Huang AY, Xu ML, Wang GC (2012) Selection of internal control gene for expression studies in Porphyra haitanensis (Rhodophyta) at different life history stages. J Phycol 48:1040–1044
Yan XH, Aruga Y (1997) Induction of pigmentation mutants by treatment of monospore germlings with NNG in Porphyra yezoensis Ueda (Bangiales, Rhodophyta). Algae 12:39–54
Yan XH, Lv F, Liu CJ, Zheng YF (2010) Selection and characterization of a high-temperature tolerant strain of Porphyra haitanensis Chang et Zheng (Bangiales, Rhodophyta). J Appl Phycol 22:511–516
Yocum CS, Blinks LR (1958) Light-induced efficiency and pigment alterations in red algae. J Gen Physiol 41:1113–1117
Zhang CC, Zhou CZ, Burnap RL, Ling P (2018) Carbon/Nitrogen metabolic balance: lessons from cyanobacteria. Trends Plant Sci 23:1116–1130
Zheng ZL (2009) Carbon and nitrogen nutrient balance signaling in plants. Plant Signal Behav 4:584–591
Zhong CH, Aruga Y, Yan XH (2019) Morphogenesis and spontaneous chromosome doubling during the parthenogenetic development of haploid female gametophytes in Pyropia haitanensis (Bangiales, Rhodophyta). J Appl Phycol 31:2729–2741
Funding
The study was supported by National Key Research and Development Program of China (2018YFD0900606), China Agriculture Research System of MOF and MARA, Science and Technology Program of Fujian Province (2019R1013-8; 2019NJJ009), and Youth Innovation Fund of Xiamen (3502Z20206001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Supplemental Fig.S1
Absolute spectral features of different light qualities. a, b, c, d represent absolute spectrum of green light (GL), blue light (BL), red light (RL), white light(WL), respectively. (PNG 11957 kb)

Supplemental Fig.S2
Observations on thallus cells of P. haitanensis under light microscope before and after experimental treatments. a, b, c and d represent thallus cells before experimental treatments, respectively; e, f, g and h represent WL-, BL-, GL- and RL-acclimated thallus cells, respectively. (PNG 5401 kb)

Supplemental Fig.S3
qPCR validation of randomly selected DEGs from transcriptome sequencing results. (PNG 18352 kb)
Supplementary Tab.S1:
Primer sequences used for qPCR validation of DEGs of P. haitanensis under different light qualities. (DOCX 13 kb)
Supplementary Tab.S2:
Statistics of DEGs of P. haitanensis under different light qualities. (DOCX 13 kb)
Supplementary Tab.S3:
GO enrichment analysis of DEGs of P. haitanensis under different light qualities. (DOCX 19 kb)
Supplementary Tab.S4:
KEGG enrichment analysis of DEGs of P. haitanensis under different light qualities. (DOCX 16 kb)
Supplementary Tab.S5:
Data of expression levels of genes encoding light-harvest protein. (DOCX 14 kb)
Supplementary Tab.S6:
Data of expression levels of genes encoding enzymes in nitrogen assimilation. (DOCX 13 kb)
Supplementary Tab.S7:
Data of expression levels of genes encoding enzymes of the Calvin cycle. (DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Liang, X., Zhong, C., Tang, L. et al. Exploration on long-term acclimation of Pyropia haitanensis thalli to monochromatic lights based on physiological characteristics and transcriptome analysis. J Appl Phycol 34, 565–576 (2022). https://doi.org/10.1007/s10811-021-02626-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02626-6