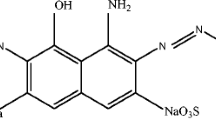
Abstract
Among existing water treatment methods for organic-containing wastewaters, advanced oxidation process, particularly electrocatalytic oxidation, is a technique allowing to reach high degradation and mineralization efficiencies. Electrodes tested for use in electrocatalytic oxidation processes contain either expensive or platinum/group metals such as Pt-, Ru-, Ir-, Pd-, or boron-doped diamond and Sb and Pb compounds which are toxic for the environment. Thereby, there is a need for environmentally friendly and less expensive electrodes. The objectives of this research were to optimize annealing temperature of Ti/Ta2O5–SnO2 electrodes, establish the working media for organic compound oxidation processes as well as check degradation, mineralization and current efficiencies for methylene blue dye oxidation. Decolorisation efficiency of 95 % was achieved in 2 h at pH = 6.5. Neutral media showed also higher efficiency towards COD decrease which was equal to 85 % after 2 h of electrolysis. The lowest energy consumption of 7.7 kWh m−3 required for 100 % decolorisation was observed for the electrodes annealed at 550 °C at pH = 2. The highest current efficiency of 10.1 % attributed to 80 % of COD reduction was obtained for the electrode annealed at 550 °C at pH = 6.5. The optimization data allow further extrapolating of electrocatalytic oxidation process on Ti/Ta2O5–SnO2 electrodes to pilot scale.
Graphical Abstract







Similar content being viewed by others
References
Beck M, Walker RV (2013) On water security, sustainability, and the water-food-energy-climate nexus. Front Environ Sci Eng 7:626–639
El-Shahawi M, Hamza A, Bashammakh A, Al-Saggaf W (2010) An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta 80:1587–1597
Pagga U, Brown D (1986) The degradation of dyestuff: part II behaviour of dyestuff in aerobic biodegradation tests. Chemosphere 15:479–491
Comninellis C (1994) Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for wastewater treatment. Electrochim Acta 39:1857–1862
Fan J, Zhao G, Zhao H, Chai S, Cao T (2013) Fabrication and application of mesoporous Sb-doped SnO2 electrode with high specific surface in electrochemical degradation of ketoprofen. Electrochim Acta 94:21–29
Palma-Goyes RE, Silva-Agredo J, González I, Torres-Palma RA (2014) Comparative degradation of indigo carmine by electrochemical oxidation and advanced oxidation processes. Electrochim Acta 140:427–433
El-Ghenymy A, Centellas F, Garrido JA, Rodríguez RM, Sirés I, Cabot PL, Brillas E (2014) Decolorization and mineralization of Orange G azo dye solutions byanodic oxidation with a boron-doped diamond anode in divided and undivided tank reactors. Electrochim Acta 130:568–576
Aquino JM, Rocha-Filho R, Ruotolo L, Bocchi N, Biaggio S (2014) Electrochemical degradation of a real textile wastewater using b-PbO2 and DSA® anodes. Chem Eng J 251:138–145
Shao D, Liang J, Cui X, Xu H, Yan W (2014) Electrochemical oxidation of lignin by two typical electrodes: Ti/Sb–SnO2 and Ti/PbO2. Chem Eng J 244:288–295
Da Silva L, Franco D, De Faria L, Boodts J (2004) Surface, kineticsand electrocatalytic properties of Ti/(IrO2 + Ta2O5) electrodes, prepared using controlled cooling rate, for ozone production. Electrochim Acta 49:3977–3988
Xu L, Xin Y, Wang J (2009) A comparative study on IrO2–Ta2O5coated titanium electrodes prepared with different methods. Electrochim Acta 54:1820–1825
Kong J, Shi S, Zhu X, Ni J (2007) Effect of Sb dopant amount on the structure and electrocatalytic capability of Ti/Sb–SnO2 electrodes in the oxidation of 4-chlorophenol. J. Environ. Sci. 19:1380–1386
Adams B, Tian M, Chen A (2009) Design and electrochemical study of SnO2-based mixed oxide electrodes. Electrochim Acta 54:1491–1498
Yue L, Wang L, Shi F, Guo J, Yang J, Lian J, Luo X (2015) Application of response surface methodology to the decolorization by the electrochemical process using FePMo12O40 catalyst. J Ind Eng Chem 21:971–979
Zhou M, Särkkä H, Sillanpää M (2011) A comparative experimental study on methyl orange degradation by electrochemical oxidation on BDD and MMO electrodes. Sep Purif Technol 78:290–297
García-Cruz L, Sáez A, Ania CO, Solla-Gullón J, Thiemann T, Iniesta J, Montiel V (2014) Electrocatalytic activity of Ni-doped nanoporous carbons in the electrooxidation of propargyl alcohol. Carbon 73:291–302
Gan T, Hu C, Chen Z, Hu S (2011) Novel electrocatalytic system for the oxidation of methyl jasmonate based on layer-by-layer assembling of montmorillonite and phosphotungstic acid nanohybrid on graphite electrode. Electrochim Acta 56:4512–4517
Zhao Y, Li C, Zhao W, Du Q, Chi B, Sun J, Chai Z, Wang X (2013) Electrocatalytic oxidation of ascorbic acid on a lithium-doped tantalum oxide film coated electrode. Electrochim Acta 107:52–58
Wang Y, Liu Q, Qi Q, Ding J, Gao X, Zhang Y, Sun Y (2013) Electrocatalytic oxidation and detection of N-acetylcysteine based onmagnetite/reduced graphene oxide composite-modified glassy carbon electrode. Electrochim Acta 111:31–40
Ghonim A, El-Anadouli B, Saleh M (2013) Electrocatalytic glucose oxidation on electrochemically oxidized glassy carbon modified with nickel oxide nanoparticles. Electrochim Acta 114:713–719
Kim J, Lee J, Tak Y (2009) Relationship between carbon corrosion and positive electrode potential in a proton-exchange membrane fuel cell during start/stop operation. J Power Sources 192:674–678
Shestakova M, Bonete P, Gómez R, Sillanpää M, Tang WZ (2014) Novel Ti-Ta2O5–SnO2 electrodes for water electrolysis and electrocatalytic oxidation of organics. Electrochim Acta 120:302–307
Comninellis C, Vercesi G (1991) Problems in DSA® coating deposition by thermal decomposition. J Appl Electrochem 21:136–142
Martínez-Hutile C, Brillas E (2009) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal B-Environ 87:105–145
Greenwood N, Earnshaw A (1997) Chemistry of the Elements, 2nd edn. Elsevier Butterworth-Heinemann, Oxford
Barsan MM, Pinto EM, Florescu M, Brett CMA (2009) Development and characterization of a new carbon composite electrode. Anal Chim Acta 635:71–78
Gu BK, Ismail YA, Spinks GM, Kim SI, So I, Kim SJ (2009) A linear actuation of polymeric nanofibrous bundle for artificial muscles. Chem Mater 21:511–515
Rivero EP, Rivera FF, Cruz-Díaz MR, Mayen E, González I (2012) Numerical simulation of mass transport in a filter press type electrochemical reactor FM01-LC: comparison of predicted and experimental mass transfer coefficient. Chem Eng Res Des 90:1969–1978
Ameur ZO, Husein MM (2013) Electrochemical behavior of potassium ferricyanide in aqueous and (w/o) microemulsion systems in the presence of dispersed nickel nanoparticles. Sep Sci Technol 48:681–689
Menolasina S (2005) Electrochemical studies of Fe(CN)64-/Fe(CN)63- on gold ultramicroelectrodes varying the concentrations of KF as supporting electrolyte. Rev Téc Ing Univ Zulia 28:159–168
Trasatti S (1987) Oxide/aqueous solution interfaces, interplay of surface chemistry and electrocatalysis. Mater Chem Phys 16:157–174
Toyosaki H, Kawasaki M, Tokura Y (2008) Electrical properties of Ta-doped SnO2 thin films epitaxially grown on TiO2 substrate. Appl Phys Lett. 93:132109-1–132109-3
Pletcher D, Walsh F (1990) Industrial electrochemistry. Springer Science & Business Media, New York, p 95
Shestakova M, Vinatoru M, Mason TJ, Sillanpää M (2015) Sonoelectrocatalytic decomposition of methylene blue using Ti/Ta2O5–SnO2 electrodes. Ultrason Sonochem 23:135–141
Kötz R, Stucki S, Carcer B (1991) Electrochemical waste water treatment using high overvoltage anodes. Part I: physiscal and electrochemical properties of SnO2 anodes. J Appl Electrochem 21:14–20
Stucki S, Kötz R, Carcer B, Suter W (1991) Electrochemical waste water treatment using high overvoltage anodes. Part II: anode performance and applications. J Appl Electrochem 21:99–104
Andrade L, Ruotolo L, Rocha-Filho R, Bocchi N, Biaggio S, Iniesta J, García-Garcia V, Montiel V (2007) On the performance of Fe and Fe, F doped Ti–Pt/PbO2 electrodes in the electrooxidation of the Blue Reactive 19 dye in simulated textile wastewater. Chemosphere 66:2035–2043
López-Grimau V, Gutiérrez M (2006) Decolourisation of simulated reactive dyebath effluents by electrochemical oxidation assisted by UV light. Chemosphere 62:106–112
Cameselle C, Pazos M, Sanromán M (2005) Selection of an electrolyte to enhance the electrochemical decolourisation of indigo. Optimisation and scale-up. Chemosphere 60:1080–1086
Andrade L, Rocha-Filho R, Bocchi N, Biaggio S, Iniesta J, García-Garcia V, Montiel V (2008) Degradation of phenol using Co- and Co, F-doped PbO2 anodes in electrochemical filter-press cells. J Hazard Mater 153:252–260
Rajkumar D, Kim J, Palanivelu K (2005) Indirect electrochemical oxidation of phenol in the presence of chloride for wastewater treatment. Chem Eng Technol 28:98–105
Yavuz Y, Koparal A (2006) Electrochemical oxidation of phenol in a parallel plate reactor using ruthenium mixed metal oxide electrode. J Hazard Mater 136:296–302
Acknowledgments
This work was supported by Maj and Tor Nessling Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shestakova, M., Graves, J., Sitarz, M. et al. Optimization of Ti/Ta2O5–SnO2 electrodes and reaction parameters for electrocatalytic oxidation of methylene blue. J Appl Electrochem 46, 349–358 (2016). https://doi.org/10.1007/s10800-016-0925-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-016-0925-5