Abstract
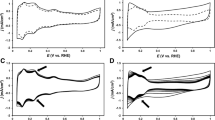
The stability of Pt–Co/C and Pt–Pd/C electrocatalysts relative to that of a commercial Pt/C catalyst was measured in terms of the loss of the electrochemical surface area (ESA). The electrocatalytic activity was investigated in an acidic solution (0.3 M H2SO4) and in a single PEM fuel cell under H2/O2 conditions. In the acidic solution, the ESA of the catalyst decreased as the number of repeated potential cycles increased, which is likely to be due to dissolution of the different metals contained within the catalyst structure. In the fuel cell environment, the deterioration of the cell performance increased as the number of repeated potential cycles increased. Thus, the loss of cell performance may be related to the loss of the ESA. In addition, the loss of the catalyst’s ESA affected the cell performance at low-, medium-, and high- current densities, indicating a loss of either the activation potential or an ohmic loss. Among the three electrocatalysts evaluated, the Pt–Co/C based one exhibited the highest electrocatalytic activity in both the acidic solution and in the fuel cell environment.







Similar content being viewed by others
References
Shinichi H, Jumbom K, Supramaniam S (1997) Electrochim Acta 4(10):1587
Bron M, Bogdanoff P, Fiechter S, Hilgendorff M, Radnik J, Dorbandt I, Schulenburg H, Tributsh H (2001) J Electroanal Chem 517(1–2):85
Bezerra CWB, Zhang L, Liu H, Lee K, Marques ALB, Marques EP, Wang H, Zhang J (2007) J Power Source 173(2):891
Bar-On I, Kirchain R, Roth R (2002) J Power Source 109:71
A.D. Little, Inc. (ADL), (2011) Cost analysis of fuel cell system for transportation: baseline system cost estimate, prepared by E.J. Carlson and S. Mariano. http://www.ott.doe.gov/pdfs/baseline_cost_model.pdf. Accessed 11 Aug 2011
Lomax FD Jr, James BD, Baum GN, Thomas CE (1997) Detailed manufacturing cost estimates for polymer electrolyte membrane (PEM) fuel cell for light duty vehicles. Directed Technologies Inc, Arlington
Vante NA, Tributsch H (1986) Nature 323:431
Fernández JL, Raghuveer V, Manthiram A, Bard AJ (2005) J Am Chem Soc 127:13100
Bagotsky VS (2009) Fuel cells, problems and solution, Chap 2. Wiley, New York
Bashyam R, Zelenay P (2006) Nature 443:63
Zelenay P (2009) Advanced cathode catalysts, Hydrogen program annual merit review and peer evaluation meeting. Arlington, Virginia, May 18–22
Ma Y, Zhang H, Zhong H, Xu T, Jin H, Tang Y, Xu Z (2010) Electrochim Acta 55(27):7945
Kim SH, Pitsch H (2009) J Electrochem Soc 156(6):B673
Mukherjee PP, Wang CY (2007) J Electrochem Soc 154(11):B1121
Wang G, Mukherjee PP, Wang CY (2006) Electrochim Acta 51(15):3139
Wang G, Mukherjee PP, Wang CY (2006) Electrochim Acta 51(15):3151
Yoon YG, Yang TH, Park GG, Lee WY, Kim CS (2003) J Power Source 118(1–2):189
Song D, Wang Q, Zhongsheng LZ, Navessin T, Holdcroft S (2004) Electrochim Acta 50(2–3):731
Wang Y, Feng X (2009) J Electrochem Soc 156(3):B403
He T, Kreidler E, Xiong L, Luo J, Zhong CJ (2006) J Electrochem Soc 153:A1637
Luo J, Kariuki N, Han L, Wang L, Zhong CJ, He T (2006) Electrochim Acta 51(23):4821
Han KH, Moon YS, Han OH, Hwang KJ, Kim I, Kim H (2007) Electrochem Commun 9(2):317
Colón-Mercado HR, Popov PN (2006) J Power Source 155(2):253
Zignani SC, Antolini E, Gonzalez ER (2008) J Power Source 182(1):83
Wu H, Wexler D, Wang G (2009) J Alloy Comp 488:195
Cho YH, Jeon TY, Lim JW, Cho YH, Ahnb M, Jung N, Yoo SJ, Yoon WS, Sung YE (2011) Int J Hydrogen Energy 36:4394
Antolini E, Salgado JRC, Giz MJ, Gonzalez ER (2005) Int J Hydrogen Energy 30(11):1213
Min M, Cho J, Cho K, Kim H (2000) Electrochim Acta 45:4211
Yang H, Vogel W, Lamy C, Alonso-Vante NC (2004) J Phys Chem B 108:11024
Xiong L, Manthiram A (2005) J Electrochem Soc 152:A697
Trongchuankij W, Poochinda K, Pruksathorn K, Hunsom M (2010) Renew Energy 35:2839
Thanasilp S, Hunsom M (2011) Electrochim Acta 56(3):1164
Thanasilp S, Hunsom M (2010) Fuel 89(12):3847
Lopes T, Antolini E, Gonzalez ER (2008) Int J Hydrogen Energy 33:5563
Xu JB, Zhao TS, Yang WW, Shen SY (2010) Int J Hydrogen Energy 35:8699
Vassos BH, Ewing GW (1983) Electroanalytical chemistry. Wiley, USA
Van Der Klink JJ (1999) Adv Catal 44:1
Lv H, Mu S, Cheng N, Pan M (2010) Appl Catal B 100:190
Huang SY, Ganesan P, Popov BN (2011) Appl Catal B 102:71
Colón-Mercado HR, Kim H, Popov BN (2004) Electrochem Com 6(8):795
Paulus UA, Wokaun A, Scherer GG, Schmidt TJ, Stamenkovic V, Markovic NM, Ross PN (2002) Electrochim Acta 47:3787
Yin S, Mu S, Lv H, Cheng N, Pan M, Fu Z (2010) Appl Catal B 93:233
Gojkovic SL, Zecevic SK, Savinell RF (1998) J Electrochem Soc 145:3712
Ralph TR, Hogarth MP (2002) Platin Met Rev 46:3
Yu P, Pemberton M, Plasse P (2005) J Power Source 144:11
Gasteiger HA, Kocha SS, Sompalli B, Wagner FT (2005) Appl Catal B 56:9
Cho YH, Choi B, Cho YH, Park HS, Sung YE (2007) Electrochem Commun 9(3):378
Prentice G (1991) Electrochemical engineering principles. Prentice Hall Inc., New Jersey
Lobato J, Cañizares P, Rodrigo MA, Linares JJ (2007) Electrochim Acta 52(12):3910
Huang SY, Ganesan P, Popov BN (2010) Appl Catal B 96:224
Ticianelli EA, Derouin CR, Redondo A, Srinivasan S (1988) J Electrochem Soc 135:2209
Song C, Zhang J (2008) Electrocatalytic oxygen reduction reaction. In: Zhang J (ed) PEM fuel cell electrocatalysts and catalyst layers: fundamentals and applications. Springer, London
Acknowledgments
The authors would like to thank the Faculty of Science, Chulalongkorn University, for financial support. The Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission (EN276B) plus the Thai Government Stimulus Package 2 (TKK2555), under the Project for Establishment of a Comprehensive Center for Innovative Food, Health Products and Agriculture are thanked for facility support. Also, we thank the Publication Counseling Unit (PCU) of the Faculty of Science, Chulalongkorn University, and Dr. Robert D.J. Butcher for comments, suggestions and checking the grammar.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Termpornvithit, C., Chewasatn, N. & Hunsom, M. Stability of Pt–Co/C and Pt–Pd/C based oxygen reduction reaction electrocatalysts prepared at a low temperature by a combined impregnation and seeding process in PEM fuel cells. J Appl Electrochem 42, 169–178 (2012). https://doi.org/10.1007/s10800-012-0384-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-012-0384-6