Abstract
Purpose
To analyze refractive and topographic changes secondary to Descemet membrane endothelial keratoplasty (DMEK) in pseudophakic eyes with Fuchs’ endothelial dystrophy (FED).
Methods
Eighty-seven pseudophakic eyes of 74 patients who underwent subsequent DMEK surgery for corneal endothelial decompensation and associated visual impairment were included. Median post-operative follow-up time was 12 months (range: 3–26 months). Main outcome measures were pre- and post-operative manifest refraction, anterior and posterior corneal astigmatism, simulated keratometry (CASimK) and Q value obtained by Scheimpflug imaging. Secondary outcome measures included corrected distance visual acuity (CDVA), central corneal densitometry, central corneal thickness, corneal volume (CV), anterior chamber volume (ACV) and anterior chamber depth (ACD).
Results
After DMEK surgery, mean pre-operative spherical equivalent (± SD) changed from + 0.04 ± 1.73 D to + 0.37 ± 1.30 D post-operatively (p = 0.06). CDVA, proportion of emmetropic eyes, ACV and ACD increased significantly during follow-up. There was also a significant decrease in posterior corneal astigmatism, central corneal densitometry, central corneal thickness and corneal volume over time (p = 0.001). Only anterior corneal astigmatism and simulated keratometry (CASimK) remained fairly stable after DMEK.
Conclusion
Despite tendencies toward a hyperopic shift, changes in SE were not significant and refraction remained overall stable in pseudophakic patients undergoing DMEK for FED. Analysis of corneal parameters by Scheimpflug imaging mainly revealed changes in posterior corneal astigmatism pointing out the relevance of posterior corneal profile changes during edema resolution after DMEK.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Currently, corneal endothelial decompensation secondary to Fuchs’ endothelial dystrophy (FED) or pseudophakic bullous keratopathy (BKP) can be treated successfully by endothelial keratoplasty (i.e., Descemet membrane endothelial keratoplasty, DMEK) [1]. DMEK, which is focused on the selective replacement of a patient’s Descemet’s membrane and the damaged corneal endothelium, has some advantages compared to penetrating keratoplasty (PKP), like faster visual rehabilitation, superior visual outcome and lower rejection rate [2,3,4,5]. Lower rates of postoperative glaucoma and easier control of intraocular pressure are further advantages compared to penetrating keratoplasty [6]. Usually a high percentage of patients seeking DMEK surgery demonstrate cataractous changes of the crystalline lens as well or are already pseudophakic at the time of surgery. Accurate prediction of the refractive outcome in patients undergoing DMEK surgery is still an unsolved problem, especially patients with combined DMEK surgery and cataract surgery (triple procedure), who often times experience an unpredictable post-operative hyperopic shift [7,8,9,10]. Currently, it is thought that changes to the posterior corneal profile due to resolution of the stroma edema may have the highest impact on the refraction outcome [11]. To better understand these changes, assessment of the posterior corneal profile, best represented by the Q value, is assumed to be the most useful in estimating and understanding the postoperative hyperopic shift more precisely. Nevertheless, changes to additional topographic parameters that may also affect the postoperative refraction still need to be defined [12]. Since a high portion of DMEK patients already had previous cataract surgery, it is of interest to better understand if and how the final refractive outcome might be affected by postoperative corneal changes in these patients first since triple procedure patients have more complicated variables to consider and evaluate.
The purpose of our study was to evaluate refractive and corneal changes in pseudophakic patients undergoing DMEK surgery for FED. Different topographic parameters were analyzed regarding their potential contribution on post-operative refractive result in DMEK patients.
Patients and methods
Inclusion criteria
Medical records of 185 consecutive pseudophakic patients, who underwent DMEK surgery for FED between February 2015 and July 2018 at our department, were reviewed retrospectively. The study protocol was approved by the institutional review board of the Goethe-University and was in accordance with the tenets of the Declaration of Helsinki.
All DMEK surgeries were performed by two experienced surgeons (TK and IS) following the protocol by Melles [2]. Inclusion criteria were a minimum follow-up time of 3 months after DMEK, stable postoperative refraction, existence of pre- and post-operative Scheimpflug images and absence of previous corneal surgery.
Pre- and post-DMEK assessment
Demographic data, CDVA, target refraction after previous cataract surgery and ocular comorbidities were extracted from medical records. Central corneal thickness (CCT), corneal astigmatism, average keratometric readings of the anterior (KmF) and posterior surface (KmB), corneal volume, posterior Q-value, corneal densitometry and anterior chamber depth and volume were evaluated using a rotating Scheimpflug camera system (Pentacam AXL, Oculus GmbH, Wetzlar, Germany).
Corneal astigmatism assessment included magnitude and axis orientation at the front and back surface as well as simulated keratometry (CASimK), a value for estimation of total corneal astigmatism determined by anterior corneal measurement only.
For analysis of the distribution of axis orientations of the corneal astigmatism, the following definitions described by Kamiya and co-workers were used:
-
Anterior astigmatism: with-the-rule (WTR: steep meridian within 60–120°), against-the-rule (ATR: steep meridian within 0–30° or 150–180°) and oblique (steep meridian within 30–60° or 120–150°).
-
Posterior astigmatism: WTR (steep meridian within 0–30° or 150–180°), ATR (steep meridian within 60–120°), the remaining astigmatism was classified as oblique astigmatism [13].
Posterior Q value, which describes the eccentricity of the posterior surface profile, can be positive (oblate cornea), zero (spheric cornea) or negative (prolate cornea). In the presence of stromal edema, the cornea tends to become more oblate (i.e., flatter centrally than peripherally, positive Q value).
Corneal densitometry, a parameter of corneal transparency, was assessed by corneal backscattered light measurements (grayscale units—GSU) of the entire cornea within three concentric corneal annular zones (0–2 mm zone, 2–6 mm zone, and 6–10 mm zone). Values ranged from 0 (completely transparent) to 100 (completely opaque).
Statistical analysis
Statistical analysis was performed using Excel for Mac (version 15.37, Microsoft, Inc., Redmond, WA, USA) and SPSS version 25 (IBM Corp., Armonk, NY, USA). Normal distribution testing was done by using the Kolmogorov–Smirnov test. For normally distributed data, a Student´s t-test for paired values was performed. A two-sided Wilcoxon signed-rank test was used for a non-normal distribution. A p-value < 0.05 was considered statistically significant.
Results
Inclusion criteria were met by 87 eyes of 74 patients (36 males and 38 females). The mean age (± SD) at the time of DMEK surgery was 71.7 ± 8.2 years (range: 47–91 years). Median follow-up time was 12 months (range: 3–26 months). The median time interval between previous cataract surgery and DMEK was 12 months (range: 2–112 months). Changes in postoperative CDVA (logMAR), refraction (spherical equivalent—SE), corneal topography, densitometry and anterior chamber are summarized in Table 1. The rebubbling rate was 17% (n = 15 eyes). Eyes with severe extracorneal visual limitations (amblyopia, macular degeneration or advanced glaucoma) were excluded from the statistical analysis of CDVA (n = 7).
After DMEK surgery, CDVA (logMAR) improved significantly from 0.68 ± 0.66 to 0.30 ± 0.29 at the end of observation period (p < 0.001).
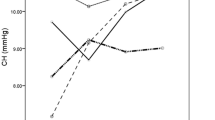
Preoperatively, the majority of study eyes (39%) were emmetropic with a spherical equivalent (SE) ranging between − 0.50 and + 0.50 diopters (D) as shown in Fig. 1. The percentage of myopic (< − 0.50 D) and hyperopic (> 0.50 D) eyes was 35% and 26%, respectively. After DMEK surgery, mean SE (± SD) increased from + 0.04 ± 1.73 D to + 0.37 ± 1.30 D at the final follow-up visit. Although there was a slight hyperopic shift in all three subgroups, the total refractive changes were statistically not significant (p = 0.06). Overall, the percentage of myopic eyes (SE < −0.5 D) decreased from 35 to 20%. In contrast, the percentage of emmetropic and hyperopic eyes increased by 8% (39% to 47%) and 7% (26% to 33%), respectively (Fig. 1). CDVA and SE demonstrated changes up to 6 months after DMEK surgery and remained relatively stable afterwards (Figs. 2 and 3).
Topographic parameters, such as anterior corneal astigmatism and simulated keratometry (CASimK) did not show any significant changes (Table 1). With-the-rule astigmatism (WTR) was present in the majority of eyes (53.2%). Postoperative changes were primarily limited to the posterior corneal surface only. For example, posterior corneal astigmatism decreased from + 0.59 ± 0.56 to + 0.39 ± 0.27 D (p = 0.001). In addition, the proportion of eyes demonstrating an against-the-rule (ATR) astigmatism increased from 42.8 to 73.6%, while the amount eyes with WTR and oblique astigmatism decreased after DMEK. Changes in the distribution of axis orientations of anterior and posterior corneal astigmatism are also shown in Fig. 4.
There was also a significant reduction in the corneal volume after DMEK (p < 0.001). Simultaneously, anterior chamber depth and volume increased significantly (Table 1). On the other hand, KmF remained fairly stable after surgery (p = 0.96). In contrast, KmB and CCT changed significantly (p < 0.001), with an increase (KmB) from −5.72 ± 0.83 to −6.33 ± 0.31 D (p < 0.001) and a decrease (CCT) from 667.9 ± 117.5 to 523.3 ± 43.6 µm (p < 0.001), respectively.
Corneal densitometry showed a significant reduction for the total layer in the 0–2 mm and 2–6 mm corneal annular zones (p < 0.001), while changes in the peripheral 6–10 mm zone were statistically not significant (p = 0.27).
Discussion
In this study, we analyzed pseudophakic eyes for potential postoperative refractive and topographic changes secondary to DMEK surgery for FED. Currently, there are only few data available focusing on the refractive outcome in patients with previous cataract surgery. As optimization of refractive results is becoming more and more relevant for patients undergoing corneal surgery, additional data are helpful for a good surgical management[7]. Furthermore, only minor refractive changes after DMEK surgery in pseudophakic eyes can be an argument in favor for a sequential management (1st step: cataract extraction; 2nd step: DMEK surgery) over triple procedure, which has a reportedly high likelihood of a clinical relevant hyperopic shift [8,9,10, 16].
Overall, we also found a hyperopic shift in pseudophakic eyes after DMEK surgery; however, changes were only minor and statistically not significant. Our results were comparable with a study performed by van Dijk et al. which analyzed the refractive outcome of pseudophakic eyes after DMEK over a period of 2 years [14]. The authors were able to demonstrate that major changes were related to the anterior corneal curvature, showing slight changes over time. In contrast to the aforementioned study, the refractive astigmatism, anterior astigmatism and KmF remained almost stable in our study cohort.
In general, prediction of the post-operative refractive outcome is still an unresolved issue in patients with DMEK surgery for FED [1, 12]. A study investigating changes in the postoperative refraction after DMEK in phakic eyes (n = 52) found a mean hyperopic shift of + 0.74 D [15]. Another study, performed by Schoenberg and colleagues, was able to detect a refractive error of + 0.43 D following triple DMEK procedures in 108 eyes [16]. The authors concluded that a target refraction of − 0.75 to − 1.00 D might be helpful in reducing the proportion of eyes with postoperative hyperopia [16]. We only partially interpret the relatively high number of study eyes being not emmetropic after previous cataract surgery (53%) as a result of the narrow definition of emmetropia (± 0.5 D). Nevertheless, the percentage of eyes with postoperative emmetropia was still higher compared to a study investigating risk factors for hyperopia after DMEK surgery with combined cataract surgery (triple DMEK procedure) (47% vs. 38%) [12]. Taking into account the individual situations in which DMEK surgery might be performed (single DMEK in phakic eyes, single DMEK in pseudophakic eyes and simultaneous DMEK and cataract surgery), the aforementioned studies and our study data suggest that hyperopic changes seem to be least pronounced after DMEK surgery in pseudophakic eyes. This is presumably because inaccurate IOL power calculation due to resolving corneal edema after DMEK surgery has no longer to be considered. These findings might be an argument for choosing a step-wise procedural protocol in patients with cataract and endothelial dystrophy or limited endothelial pump function.
As already shown in previous studies, the anterior corneal profile remains fairly stable after DMEK surgery [17]. Therefore, reasons for postoperative hyperopic changes may be mainly related to modifications of the posterior corneal profile itself [18]. This assumption could be supported by our findings in pseudophakic FED showing no statistic significant changes in the anterior corneal astigmatism and KmF after DMEK surgery. However, we observed significant changes in regard to the posterior corneal astigmatism. Our findings are in accordance with a study performed by Yokogawa et al., which also showed a significant decrease in the magnitude of the posterior corneal astigmatism after DMEK surgery [19]. Along with a reduction of the magnitude of posterior corneal astigmatism, we also detected a shift in axis orientation to a higher proportion of eyes with ATR astigmatism, which is similar to results reported by Alnawaiseh et al. [20]. In a study with non-diseased, previously unoperated eyes, total corneal astigmatism could not be predicted adequately by anterior measurements only, especially in eyes that do not have with-the-rule astigmatism, pointing out the relevance of posterior corneal profile [21]. As is known in refractive surgery, posterior corneal astigmatism is a relevant parameter in IOL calculation to improve the postoperative refractive outcome [22, 23]. Unfortunately, reliable prediction of the post-operative astigmatism based on pre-operative measurements alone is not sufficiently possible in eyes with FED yet [24].
In a previous study investigating factors contributing to hyperopic refractive outcomes in patients with combined cataract and DMEK surgery (triple procedure), there was a higher risk of hyperopic shift in FED eyes with positive pre-operative posterior Q values [12]. In our cohort, we did not observe a similar correlation despite negative posterior Q values after resolution of the stromal edema. Nevertheless, we assume that resolution of stromal edema is an important contributing factor to the hyperopic shift observed in triple DMEK due to its impact on IOL power calculation. Therefore, performing cataract surgery in FED patients prior to the development of corneal decompensation might limit the factor of inaccurate IOL power calculation.
Factors such as performance of cataract surgery at different institutions, heterogeneous time intervals between cataract surgery and DMEK surgery as well as differences concerning duration and extent of corneal decompensation represent limitations of our study. Furthermore, it has to be validated whether our findings obtained from pseudophakic patients with FED can be applied to other causes of endothelial dysfunction, like pseudophakic bullous keratopathy.
Despite a slight tendency toward a postoperative hyperopic shift, we could demonstrate that changes in SE were statistically not significant. Our analysis of corneal parameters by Scheimpflug tomography points out the relevance of changes in posterior corneal astigmatism for refractive outcome. However, an unexpected refractive outcome frequently reported in patients undergoing triple DMEK [1, 12] seems to be less common after DMEK in pseudophakic patients.
References
Deng SX, Lee WB, Hammersmith KM et al (2018) Descemet membrane endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology 125(2):295–310. https://doi.org/10.1016/j.ophtha.2017.08.015
Melles GR, Ong TS, Ververs B, van der Wees J (2006) Descemet membrane endothelial keratoplasty (DMEK). Cornea 25(8):987–990. https://doi.org/10.1097/01.ico.0000248385.16896.34
Price FW Jr, Price MO (2013) Evolution of endothelial keratoplasty. Cornea 32(Suppl 1):S28–S32. https://doi.org/10.1097/ICO.0000000000000505
Maier A-KB, Gundlach E, Gonnermann J et al (2013) Fellow eye comparison of Descemet membrane endothelial keratoplasty and penetrating keratoplasty. Cornea 32(10):1344–1348. https://doi.org/10.1097/ICO.0b013e31829dd816
Anshu A, Price MO, Price FW (2012) Risk of corneal transplant rejection significantly reduced with Descemet’s membrane endothelial keratoplasty. Ophthalmology 119(3):536–540. https://doi.org/10.1016/j.ophtha.2011.09.019
Abdelghany AA, D’Oria F, Alio JL (2021) Surgery for glaucoma in modern corneal graft procedures. Surv Ophthalmol 66(2):276–289
Price FW Jr, Price MO (2017) Combined cataract/DSEK/DMEK: changing expectations. Asia Pac J Ophthalmol 6(4):388–392. https://doi.org/10.22608/APO.2017127 (Phila)
Terry MA, Shamie N, Chen ES, Phillips PM, Shah AK, Hoar KL, Friend DJ (2009) Endothelial keratoplasty for Fuchs’ dystrophy with cataract: complications and clinical results with the new triple procedure. Ophthalmology 116(4):631–639. https://doi.org/10.1016/j.ophtha.2008.11.004
Laaser K, Bachmann BO, Horn FK, Cursiefen C, Kruse FE (2012) Descemet membrane endothelial keratoplasty combined with phacoemulsification and intraocular lens implantation: advanced triple procedure. Am J Ophthalmol 154(1):47–55. https://doi.org/10.1016/j.ajo.2012.01.020
Röck T, Bartz-Schmidt KU, Röck D, Yoeruek E (2014) Refractive changes after Descemet membrane endothelial keratoplasty. Ophthalmologe 111(7):649–653. https://doi.org/10.1007/s00347-013-2939-2
Alnawaiseh M, Zumhagen L, Rosentreter A, Eter N (2017) Intraocular lens power calculation using standard formulas and ray tracing after DMEK in patients with Fuchs endothelial dystrophy. BMC Ophthalmol 17(1):152. https://doi.org/10.1186/s12886-017-0547-7
Fritz M, Grewing V, Böhringer D, Lapp T, Maier P, Reinhard T, Wacker K (2019) avoiding hyperopic surprises after Descemet membrane endothelial keratoplasty in fuchs dystrophy eyes by assessing corneal shape. Am J Ophthalmol 197:1–6. https://doi.org/10.1016/j.ajo.2018.08.052
Kamiya K, Shimizu K, Igarashi A, Miyake T (2015) Assessment of anterior, posterior, and total central corneal astigmatism in eyes with keratoconus. Am J Ophthalmol 160:851–857. https://doi.org/10.1016/j.ajo.2015.08.016
van Dijk K, Rodriguez-Calvo-de-Mora M, van Esch H, Frank L, Dapena I, Baydoun L, Oellerich S, Melles GR (2016) Two-year refractive outcomes after Descemet membrane endothelial keratoplasty. Cornea 35(12):1548–1555
Parker J, Dirisamer M, Naveiras M et al (2012) Outcomes of Descemet membrane endothelial keratoplasty in phakic eyes. J Cataract Refract Surg 38(5):871–877. https://doi.org/10.1016/j.jcrs.2011.11.038
Schoenberg ED, Price FW Jr, Miller J, McKee Y, Price MO (2015) Refractive outcomes of Descemet membrane endothelial keratoplasty triple procedures (combined with cataract surgery). J Cataract Refract Surg 41(6):1182–1189. https://doi.org/10.1016/j.jcrs.2014.09.042
Ham L, Dapena I, Moutsouris K et al (2011) Refractive change and stability after Descemet membrane endothelial keratoplasty. Effect of corneal dehydration-induced hyperopic shift on intraocular lens power calculation. J Cataract Refract Surg 37(8):1455–1464. https://doi.org/10.1016/j.jcrs.2011.02.033
Wacker K, McLaren JW, Patel SV (2015) Directional posterior corneal profile changes in Fuchs’ endothelial corneal dystrophy. Invest Ophthalmol Vis Sci 56:5904–5911. https://doi.org/10.1167/iovs.15-17311
Yokogawa H, Sanchez PJ, Mayko ZM, Straiko MD, Terry MA (2016) Corneal astigmatism stability in Descemet membrane endothelial keratoplasty for Fuchs corneal dystrophy. Cornea 35:932–937. https://doi.org/10.1097/ICO.0000000000000882
Alnawaiseh M, Zumhagen L, Rosentreter A, Eter N (2017) Changes in anterior, posterior, and total corneal astigmatism after Descemet membrane endothelial keratoplasty. J Ophthalmol 2017:4068963. https://doi.org/10.1155/2017/4068963
Tonn B, Klaproth OK, Kohnen T (2014) Anterior surface-based keratometry compared with Scheimpflug tomography-based total corneal astigmatism. Invest Ophthalmol Vis Sci 56(1):291–298. https://doi.org/10.1167/iovs.14-15659
Ho J-D, Tsai C-Y, Liou S-W (2009) Accuracy of corneal astigmatism estimation by neglecting the posterior corneal surface measurement. Am J Ophthalmol 147:788–795. https://doi.org/10.1016/j.ajo.2008.12.020
Koch DD, Jenkins RB, Weikert MP, Yeu E, Wang L (2013) Correcting astigmatism with toric intraocular lenses: effect of posterior corneal astigmatism. J Cataract Refract Surg 39:1803–1809. https://doi.org/10.1016/j.jcrs.2013.06.027
Shajari M, Kolb CM, Mayer WJ, Agha B, Steinwender G, Dirisamer M, Priglinger S, Kohnen T, Schmack I (2019) Characteristics of preoperative and postoperative astigmatism in patients having Descemet membrane endothelial keratoplasty. J Cataract Refract Surg 45(7):1001–1006. https://doi.org/10.1016/j.jcrs.2019.02.002
Funding
Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. Prof. Thomas Kohnen is a consultant to and conducts research for J&J Vision (Abbott Medical Optics, Inc.), Novartis (Alcon Laboratories, Inc.), Oculentis GmbH, Avedro Inc., Oculus Optikgeräte GmbH, Presbia, Schwind Eye-Tech Solutions GmbH and Carl Zeiss Meditec AG; serves as a consultant for Allergan Inc., Bausch & Lomb, Dompé, Geuder AG, Med Update GmbH, Rayner, Santen GmbH, STAAR Surgical AG, Thea Pharma GmbH, TearLab Corp., Thieme Compliance GmbH, Ziemer Opthalmology GmbH; and has received research funding from Hoya Surgical Optics GmbH.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, analysis and interpretation of data. Material preparation and data collection were performed by BA, NA, TK and IS. The first draft of the manuscript was written by BA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The following authors have no financial disclosure: B. Agha, N. Ahmad, D. Dawson and I. Schmack. All authors attest that they meet the current ICMJE criteria for authorship. The authors have no conflicting interests.
Ethical approval
The study was approved by the Ethics Committee of Goethe University Frankfurt (approval number 126/19). This study complies with the tenets of the 1964 Declaration of Helsinki.
Informed consent
All participants provided written informed consent indicating their permission to scientific publication of the data obtained from their treatment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Agha, B., Ahmad, N., Dawson, D.G. et al. Refractive outcome and tomographic changes after Descemet membrane endothelial keratoplasty in pseudophakic eyes with Fuchs’ endothelial dystrophy. Int Ophthalmol 41, 2897–2904 (2021). https://doi.org/10.1007/s10792-021-01850-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-01850-w