Abstract
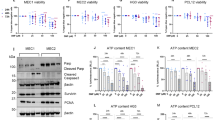
Dimethyl fumarate (DMF) is approved as a treatment for multiple sclerosis (MS), however, its mode of action remains unclear. One hypothesis proposes that Michael addition to thiols by DMF, notably glutathione is immunomodulatory. The alternative proposes that monomethyl fumarate (MMF), the hydrolysis product of DMF, is a ligand to the fatty acid receptor GPR109A found in the lysosomes of immune cells. We prepared esters of MMF and macrolides derived from azithromycin, which were tropic to immune cells by virtue of lysosomal trapping. We tested the effects of these substances in an assay of response to Lipopolysaccharide (LPS) in freshly isolated human peripheral blood mononuclear cells (PBMCs). In this system, we observed that the 4′′ ester of MMF (compound 2 and 3) reduced levels of Interleukins (IL)-1β, IL-12 and tumor necrosis factor alpha (TNFα) significantly at a concentration of 1 µM, while DMF required about 25 µM for the same effect. The 2′ esters of MMF (compound 1 and 2) were, like MMF itself, inactive in vitro. The 4′′ ester formed glutathione conjugates rapidly while the 2′ conjugates did not react with thiols but did hydrolyze slowly to release MMF in these cells. We then tested the substances in vivo using the imiquimod/isostearate model of psoriasis where the 2′ ester was the most active at 0.06–0.12 mg/kg (approximately 0.1 µmol/kg), improving skin score, body weight and cytokine levels (TNFα, IL-17A, IL-17F, IL-6, IL-1β, NLRP3 and IL-23A). In contrast, the thiol reactive 4′′ ester was less active than the 2′ ester while DMF was ca. 300-fold less active. The thiol reactive 4′′ ester was not easily recovered from either plasma or organs while the 2′ ester exhibited conventional uptake and elimination. The 2′ ester also reduced levels of IL-6 in acute monosodium urate (MSU) induced inflammation. These data suggest that mechanisms that are relevant in vivo center on the release of MMF. Given that GPR109A is localized to the lysosome, and that lysosomal trapping increases 2′ ester activity by > 300 fold, these data suggest that GPR109A may be the main target in vivo. In contrast, the effects associated with glutathione (GSH) conjugation in vitro are unlikely to be as effective in vivo due to the much lower dose in use which cannot titrate the more concentrated thiols. These data support the case for GPR109A modulation in autoimmune diseases.

Adapted from BioRender (2022) “Arm with Rash and Callout”





Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data availability
Enquiries about data availability should be directed to the authors.
References
Angiari S, O’Neill LA (2018) Dimethyl fumarate: targeting glycolysis to treat MS. Cell Res 28(6):613–615. https://doi.org/10.1038/s41422-018-0045-3
Balloy V et al (2014) Azithromycin analogue CSY0073 attenuates lung inflammation induced by LPS challenge. Br J Pharmacol 171(7):1783–1794. https://doi.org/10.1111/bph.12574
Bermudez Y, Benavente CA, Meyer RG, Coyle WR, Jacobson MK, Jacobson EL (2011) Nicotinic acid receptor abnormalities in human skin cancer: implications for a role in epidermal differentiation. PLoS One. https://doi.org/10.1371/journal.pone.0020487
Bosnar M, Kelnerić Ž, Munić V, Eraković V, Parnham MJ (2005) Cellular uptake and efflux of azithromycin, erythromycin, clarithromycin, telithromycin, and cethromycin. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.49.6.2372-2377.2005
Brennan MS et al (2015) Dimethyl fumarate and monoethyl fumarate exhibit differential effects on KEAP1, NRF2 activation, and glutathione depletion in vitro. PLoS One. https://doi.org/10.1371/journal.pone.0120254
Burnet M et al (2015) Anti-inflammatory macrolides to manage chronic neutrophilic inflammation. RSC Drug Discov Ser 201(40):206–234
Burnet MW et al. (2018) Novel anti-infective and anti-inflammatory compounds. US20200262857
Carryn S, Chanteux H, Seral C, Mingeot-Leclercq MP, Van Bambeke F, Tulkens PM (2003) Intracellular pharmacodynamics of antibiotics. Infect Dis Clin North Am. https://doi.org/10.1016/S0891-5520(03)00066-7
Chen H et al (2014) Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J Clin Invest 124(5):2188–2192. https://doi.org/10.1172/JCI72151
Dibbert S, Clement B, Skak-Nielsen T, Mrowietz U, Rostami-Yazdi M (2013) Detection of fumarate-glutathione adducts in the portal vein blood of rats: evidence for rapid dimethylfumarate metabolism. Arch Dermatol Res 305(5):447–451. https://doi.org/10.1007/s00403-013-1332-y
Gambhir D et al (2012) GPR109A as an anti-inflammatory receptor in retinal pigment epithelial cells and its relevance to diabetic retinopathy. Invest Ophthalmol Vis Sci 53(4):2208–2217. https://doi.org/10.1167/iovs.11-8447
Ghoreschi K et al (2011) Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med 208(11):2291–2303. https://doi.org/10.1084/jem.20100977
Gillard GO et al (2015) DMF, but not other fumarates, inhibits NF-κB activity in vitro in an Nrf2-independent manner. J Neuroimmunol 283:74–85. https://doi.org/10.1016/j.jneuroim.2015.04.006
Guerne P-A, Terkeltaub R, Zuraw B, Lotz M (1989) Inflammatory microcrystals stimulate interleukin-6 production and secretion by human monocytes and synoviocytes. Arthritis Rheum 32(11):1443–1452. https://doi.org/10.1002/anr.1780321114
Helwa I, Choudhary V, Chen X, Kaddour-Djebbar I, Bollag WB (2017) Anti-psoriatic drug monomethylfumarate increases nuclear factor erythroid 2-related factor 2 levels and induces aquaporin-3 mRNA and protein expression. J Pharmacol Exp Ther 362(2):243–253. https://doi.org/10.1124/jpet.116.239715
Hoyle C, Green JP, Allan SM, Brough D, Lemarchand E (2022) Itaconate and fumarate derivatives inhibit priming and activation of the canonical NLRP3 inflammasome in macrophages. Immunology 165(4):460–480. https://doi.org/10.1111/imm.13454
Huang S-W, Shieh J-J (2016) The antibiotic azithromycin improves the severity of imiquimod-induced psoriasis-like skin inflammation in mice. J Dermatol Sci 84(1):e68. https://doi.org/10.1016/j.jdermsci.2016.08.210
Humphries F et al (2020) Succination inactivates gasdermin D and blocks pyroptosis. Science (80-) 369(6511):1633–1637. https://doi.org/10.1126/science.abb9818
Kadam DP, Suryakar AN, Ankush RD, Kadam CY, Deshpande KH (2010) Role of oxidative stress in various stages of psoriasis. Indian J Clin Biochem 25(4):388–392. https://doi.org/10.1007/s12291-010-0043-9
Khameneh HJ et al (2017) C5a regulates IL-1β production and leukocyte recruitment in a murine model of monosodium urate crystal-induced peritonitis. Front Pharmacol 8(JAN):1–11. https://doi.org/10.3389/fphar.2017.00010
Kornberg MD et al (2018) Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science (80-) 360(6387):449–453. https://doi.org/10.1126/science.aan4665
Kostylina G, Simon D, Fey MF, Yousefi S, Simon HU (2008) Neutrophil apoptosis mediated by nicotinic acid receptors (GPR109A). Cell Death Differ 15(1):134–142. https://doi.org/10.1038/sj.cdd.4402238
Lehmann M et al (2002) Fumaric acid esters are potent immunosuppressants: inhibition of acute and chronic rejection in rat kidney transplantation models by methyl hydrogen fumarate. Arch Dermatol Res 294(9):399–404. https://doi.org/10.1007/s00403-002-0347-6
Lehmann JCU et al (2007) Dimethylfumarate induces immunosuppression via glutathione depletion and subsequent induction of heme oxygenase 1. J Invest Dermatol 127(4):835–845. https://doi.org/10.1038/sj.jid.5700686
Li G et al (2010) Internalization of the human nicotinic acid receptor GPR109A is regulated by Gi, GRK2, and arrestin3. J Biol Chem 285(29):22605–22618. https://doi.org/10.1074/jbc.M109.087213
Linker RA, Gold R (2013) Dimethyl fumarate for treatment of multiple sclerosis: Mechanism of action, effectiveness, and side effects. Curr Neurol Neurosci Rep. https://doi.org/10.1007/s11910-013-0394-8
Linker RA, Haghikia A (2016) Dimethyl fumarate in multiple sclerosis: latest developments, evidence and place in therapy. Ther Adv Chronic Dis 7(4):198–207. https://doi.org/10.1177/2040622316653307
Liu X et al (2016a) Dimethyl fumarate ameliorates dextran sulfate sodium-induced murine experimental colitis by activating Nrf2 and suppressing NLRP3 inflammasome activation. Biochem Pharmacol. https://doi.org/10.1016/j.bcp.2016.05.002
Liu X et al (2016b) Dimethyl fumarate ameliorates dextran sulfate sodium-induced murine experimental colitis by activating Nrf2 and suppressing NLRP3 inflammasome activation. Biochem Pharmacol 112:37–49. https://doi.org/10.1016/j.bcp.2016.05.002
Mariotte A et al (2020) A mouse model of MSU-induced acute inflammation in vivo suggests imiquimod-dependent targeting of Il-1β as relevant therapy for gout patients. Theranostics 10(5):2158–2171. https://doi.org/10.7150/thno.40650
McGuire VA et al (2016) Dimethyl fumarate blocks pro-inflammatory cytokine production via inhibition of TLR induced M1 and K63 ubiquitin chain formation. Sci Rep. https://doi.org/10.1038/srep31159
Michell-Robinson MA et al (2016) Effects of fumarates on circulating and CNS myeloid cells in multiple sclerosis. Ann Clin Transl Neurol 3(1):27–41. https://doi.org/10.1002/acn3.270
Miglio G, Veglia E, Fantozzi R (2015) Fumaric acid esters prevent the NLRP3 inflammasome-mediated and ATP-triggered pyroptosis of differentiated THP-1 cells. Int Immunopharmacol 28(1):215–219. https://doi.org/10.1016/j.intimp.2015.06.011
Mills EA, Ogrodnik MA, Plave A, Mao-Draayer Y (2018) Emerging understanding of the mechanism of action for dimethyl fumarate in the treatment of multiple sclerosis. Front Neurol. https://doi.org/10.3389/fneur.2018.00005
Mrowietz U, Morrison PJ, Suhrkamp I, Kumanova M, Clement B (2018) The pharmacokinetics of fumaric acid esters reveal their in vivo effects. Trends Pharmacol Sci 39(1):1–12. https://doi.org/10.1016/j.tips.2017.11.002
Ocana C, C Yang, M Bernal, AR Quesada, MÅ Medina (2021) The anti-angiogenic compound dimethyl fumarate inhibits the serine synthesis pathway and increases glycolysis in endothelial cells. pp. 1–78. https://doi.org/10.1101/2021.12.13.472337
Park JB, Park H, Son J, Ha SJ, Cho HS (2019) Structural study of monomethyl fumarate-bound human gapdh. Mol Cells 42(8):597–603. https://doi.org/10.14348/MOLCELLS.2019.0114
Pleńkowska J, Gabig-Cimińska M, Mozolewski P (2020) Oxidative stress as an important contributor to the pathogenesis of psoriasis. Int J Mol Sci 21(17):1–15. https://doi.org/10.3390/ijms21176206
Roberge CJ, R de Medicis, JM Dayer, M Rola-Pleszczynski, PH Naccache, PE Poubelle Crystal-induced neutrophil activation. V. Differential production of biologically active IL-1 and IL-1 receptor antagonist. J Immunol, vol. 152, no. 11, pp. 5485–5494, 1994, [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/8189066
Saxena VN, Dogra J (2010) Long-term oral azithromycin in chronic plaque psoriasis: a controlled trial. Eur J Dermatology 20(3):329–333. https://doi.org/10.1684/ejd.2010.0930
Schmidt TJ, Ak M, Mrowietz U (2007) Reactivity of dimethyl fumarate and methylhydrogen fumarate towards glutathione and N-acetyl-l-cysteine-Preparation of S-substituted thiosuccinic acid esters. Bioorganic Med Chem 15(1):333–342. https://doi.org/10.1016/j.bmc.2006.09.053
Sebök B, Bonnekoh B, Geisel J, Mahrle G (1994) Antiproliferative and cytotoxic profiles of antipsoriatic fumaric acid derivatives in keratinocyte cultures. Eur J Pharmacol Environ Toxicol 270(1):79–87. https://doi.org/10.1016/0926-6917(94)90083-3
Straß S, Schwamborn A, Keppler M, Guse J, Burnet M (2021) Synthesis, characterization and in vivo distribution of intracellular delivered macrolide SCFA derivatives. ChemMedChem 16:1–16
Sullivan LB et al (2013) The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Mol Cell 51(2):236–248. https://doi.org/10.1016/j.molcel.2013.05.003
Togami K, Chono S, Morimoto K (2013) Subcellular distribution of azithromycin and clarithromycin in rat alveolar macrophages (NR8383) in vitro. Biol Pharm Bull 36:1494. https://doi.org/10.1248/bpb.b13-00423
Wollina U (2011) Fumaric acid esters in dermatology. Indian Dermatol Online J 2(2):111. https://doi.org/10.4103/2229-5178.86007
Xu Z et al (2019) The visualization of lysosomal and mitochondrial glutathione via near-infrared fluorophore and in vivo imaging application. Sens Actuators B Chem 290(December 2018):676–683. https://doi.org/10.1016/j.snb.2019.03.114
Zheng L et al (2015) Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat Commun. https://doi.org/10.1038/ncomms7001
Acknowledgements
We would like to thank colleagues from Synovo GmbH and the University of Tübingen who assisted in this research. Special thanks to the members of the in vivo facility and the team of the analytics/bioanalytics department at both institutions.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
All authors have read and agreed to the published version of the manuscript. Conceptualization, SS, TLS and MB; methodology, SS, NC, TLS and MB; validation, SS and TLS; formal analysis, SS and TLS; investigation, JG, SS, TLS, LDO, NS, SG and AS; resources, MB and SL; writing—original draft preparation, SS; writing—review and editing, TLS, MB and JHG; visualization, SS; supervision, MB, JHG and SL; project administration, MB; funding acquisition, MB.
Corresponding author
Ethics declarations
Conflict of interest
S.S., J.G., N.C., M.M., S.G., A.S., N.S., L.D.O., J.H.G., T.L.S. are employees of Synovo GmbH. M.B. is general manager of Synovo GmbH. Synovo GmbH holds patent rights to the published structures.
Institutional review board statement
All study protocols were approved by the local Animal Care and Ethics Committee (Federal government ethics committee, Tübingen, Germany under the licenses 35/9185.81-7/SYN 06/20; 35/9185.81-7/SYN 07/18; and 35/9185.81-7/SYN 11/19). Human blood products used in the in vitro assays (used for cell stimulation, viability, stability and uptake assays) were obtained from the center for transfusion medicine in Tübingen, Germany (Zentrum für Klinische Transfusionmedizin Tübingen GmbH, (ethical approval number ZKT-FoPro202106-2305-01 and ZKT-FoPro202012-2211)).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Straß, S., Geiger, J., Cloos, N. et al. Immune cell targeted fumaric esters support a role of GPR109A as a primary target of monomethyl fumarate in vivo. Inflammopharmacol 31, 1223–1239 (2023). https://doi.org/10.1007/s10787-023-01186-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01186-0