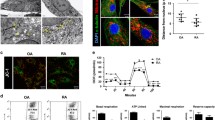
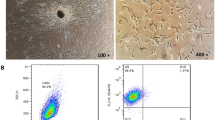
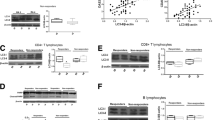
Abstract
Background
Imbalance between apoptosis and autophagy in fibroblast-like synoviocytes (FLS) is one of the pathogenic mechanisms responsible for their abnormal proliferation in rheumatoid arthritis (RA). Methotrexate (MTX) demonstrated limited efficacy in amending this imbalance in fluid-derived (fd)-FLS. The active compound of black tea Theaflavin 3,3′-digallate (TF3) may be effective in restoring apoptosis–autophagy imbalance in (fd)-FLS. The combined effect of MTX + TF3 upon the same is yet to be elucidated.
Objective
To evaluate the effect of MTX + TF3 on fd-FLS to induce apoptosis and inhibit autophagy through Endoplasmic Reticulum (ER) stress-mediated pathways.
Methods
FLS from synovial fluid of 11 RA and 10 osteoarthritis patients were cultured after treatment with MTX/TF3 or a combination of MTX (125 nM) and TF3(10 µM) and the following parameters were evaluated. C-reactive protein, cytokines (TNF-α, IL-6), angiogenic markers were quantified by ELISA. fd-FLS viability was determined by MTT assay and apoptosis by flow cytometry. ER stress markers were estimated by RT-PCR (IRE1A, spliced-XBP-1) and immunoblotting (Grp78, Hsp70, CHOP, HIF-1α). Immunoblot studies were done to evaluate apoptotic (Bcl-2, Bax, Caspases) and autophagic (Beclin1, LC3b, p62) proteins.
Results
MTX (IC25) and TF3 (IC50) both in single doses could down-regulate the levels of pro-inflammatory and angiogenic markers. Combinatorial treatment modulated autophagosomal proteins in fd-FLS and induced apoptosis by regulating ER stress response.
Conclusion
Disruption in homeostasis between apoptosis and autophagy in fd-FLS might be an underlying phenomenon in the progression of pathophysiology in RA. Co-administration of MTX + TF3 successfully restored the homeostasis by inducing apoptosis.







Similar content being viewed by others
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Abbreviations
- ANG-1:
-
Angiopoietin 1
- ACR/EULAR:
-
American College of Rheumatology/European League Against Rheumatism
- ANOVA:
-
Analysis of variance
- Caspase:
-
Cysteine-aspartic proteases
- CRP:
-
C-reactive protein
- CHOP:
-
C/EBP homologous protein
- cDNA:
-
Complementary DNA
- DMARDs:
-
Disease-modifying anti-rheumatic drugs
- DEPC:
-
Diethyl pyrocarbonate
- ELISA:
-
Enzyme-linked immunosorbent assay
- ER-Stress:
-
Endoplasmic reticulum stress
- ERAD:
-
Endoplasmic-reticulum-associated protein degradation
- FBS:
-
Fetal Bovine serum
- FITC:
-
Fluorescein isothiocyanate
- fd-FLS :
-
Fluid-derived fibroblast-like synoviocytes
- GAPDH:
-
Glyceraldehyde–3 phosphate dehydrogenase
- GRP-78:
-
78-KDa glucose-regulated protein
- HIF-1 α:
-
Hypoxia-inducible factor 1-alpha
- HSP70:
-
Heat shock protein 70
- IL-6:
-
Interleukin 6
- IRE1A:
-
Serine/threonine-protein kinase/endoribonuclease A
- LC3b:
-
Microtubule-associated proteins 1A/1B light chain 3B
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- MTX:
-
Methotrexate
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- OA:
-
Osteoarthritis
- PBS:
-
Phosphate-buffered saline
- p-62:
-
Sequestosome 1(SQSTM1)
- PCRP:
-
Polymerase chain reaction
- RA:
-
Rheumatoid Arthritis
- RT-PCR:
-
Real-time polymerase chain reaction
- RPM:
-
Revolutions per minute
- RT:
-
Room temperature
- RNA:
-
Ribonucleic acid
- RASF:
-
Rheumatoid arthritis synovial fibroblast
- SF:
-
Synovial fluid
- SD:
-
Standard deviation
- TF3:
-
Theaflavin-3,3′-digallate
- TNF-α:
-
Tumor necrosis factor-alpha
- UPR:
-
Unfolded protein responses
- VEGF:
-
Vascular endothelial growth factor
- 3-MA:
-
3-Methyladenine
- XBP-1:
-
X box-binding protein 1
References
Ahmadiany M, Alavi-Samani M, Hashemi Z et al (2019) The increased RNase activity of IRE1alpha in PBMCs from patients with rheumatoid arthritis. Adv Pharm Bull 9:505–509. https://doi.org/10.15171/apb.2019.060
Aletaha D, Neogi T, Silman AJ et al (2010) 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69:1580–1588. https://doi.org/10.1136/ard.2010.138461
Alunno A, Carubbi F, Giacomelli R, Gerli R (2017) Cytokines in the pathogenesis of rheumatoid arthritis: new players and therapeutic targets. BMC Rheumatol 1:3. https://doi.org/10.1186/s41927-017-0001-8
Andersson AM, Andersson B, Lorell C et al (2016) Autophagy induction targeting mTORC1 enhances Mycobacterium tuberculosis replication in HIV co-infected human macrophages. Sci Rep 6:28171. https://doi.org/10.1038/srep28171
Bello AE, Perkins EL, Jay R, Efthimiou P (2017) Recommendations for optimizing methotrexate treatment for patients with rheumatoid arthritis. Open Access Rheumatol 9:67–79. https://doi.org/10.2147/OARRR.S131668
Bergstrom B, Carlsten H, Ekwall AH (2018) Methotrexate inhibits effects of platelet-derived growth factor and interleukin-1beta on rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Res Ther 20:49. https://doi.org/10.1186/s13075-018-1554-7
Bustamante MF, Garcia-Carbonell R, Whisenant KD, Guma M (2017) Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther 19:110. https://doi.org/10.1186/s13075-017-1303-3
Chun Y, Kim J (2018) Autophagy: an essential degradation program for cellular homeostasis and life. Cells. https://doi.org/10.3390/cells7120278
Decuypere JP, Parys JB, Bultynck G (2012) Regulation of the autophagic bcl-2/beclin 1 interaction. Cells 1:284–312. https://doi.org/10.3390/cells1030284
Dutta A, Bandyopadhyay S, Mandal C, Chatterjee M (2005) Development of a modified MTT assay for screening antimonial resistant field isolates of Indian visceral leishmaniasis. Parasitol Int 1:119–122. https://doi.org/10.1016/j.parint.2005.01.001
Elshabrawy HA, Chen Z, Volin MV et al (2015) The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis 18:433–448. https://doi.org/10.1007/s10456-015-9477-2
Fairbanks LD, Ruckemann K, Qiu Y et al (1999) Methotrexate inhibits the first committed step of purine biosynthesis in mitogen-stimulated human T-lymphocytes: a metabolic basis for efficacy in rheumatoid arthritis? Biochem J 342(Pt 1):143–152
Gao Y, Rankin GO, Tu Y, Chen YC (2016) Theaflavin-3, 3′-digallate decreases human ovarian carcinoma OVCAR-3 cell-induced angiogenesis via Akt and Notch-1 pathways, not via MAPK pathways. Int J Oncol 48:281–292. https://doi.org/10.3892/ijo.2015.3257
Goncharova SA, Frankfurt OS (1976) Effect of methotrexate on the cell cycle of L1210 leukemia. Cell Tissue Kinet 9:333–340. https://doi.org/10.1111/j.1365-2184.1976.tb01281.x
Gosslau A, Li S, Zachariah E, Ho C-T (2018) therapeutic connection between black tea theaflavins and their benzotropolone core structure. Curr Pharmacol Rep 4:447–452. https://doi.org/10.1007/s40495-018-0157-y
Guo Q, Wang Y, Xu D et al (2018) Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res 6:15. https://doi.org/10.1038/s41413-018-0016-9
Guzel E, Arlier S, Guzeloglu-Kayisli O et al (2017) Endoplasmic reticulum stress and homeostasis in reproductive physiology and pathology. Int J Mol Sci. https://doi.org/10.3390/ijms18040792
Herman S, Zurgil N, Deutsch M (2005) Low dose methotrexate induces apoptosis with reactive oxygen species involvement in T lymphocytic cell lines to a greater extent than in monocytic lines. Inflamm Res 54:273–280. https://doi.org/10.1007/s00011-005-1355-8
Heymann D (2006) Autophagy: a protective mechanism in response to stress and inflammation. Curr Opin Investig Drugs 7:443–450
Hu H, Tian M, Ding C, Yu S (2018) The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front Immunol 9:3083. https://doi.org/10.3389/fimmu.2018.03083
Huang R, Liu W (2015) Identifying an essential role of nuclear LC3 for autophagy. Autophagy 11:852–853. https://doi.org/10.1080/15548627.2015.1038016
Jung S, Jeong H, Yu SW (2020) Autophagy as a decisive process for cell death. Exp Mol Med 52:921–930. https://doi.org/10.1038/s12276-020-0455-4
Junjappa RP, Patil P, Bhattarai KR et al (2018) IRE1α implications in endoplasmic reticulum stress-mediated development and pathogenesis of autoimmune diseases. Front Immunol 9:1289. https://doi.org/10.3389/fimmu.2018.01289
Kabala PA, Angiolilli C, Yeremenko N et al (2017) Endoplasmic reticulum stress cooperates with Toll-like receptor ligation in driving activation of rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Res Ther 19:207. https://doi.org/10.1186/s13075-017-1386-x
Kanai M, Göke M, Tsunekawa S et al (1997) Signal transduction pathway of human fibroblast growth factor receptor 3: identification of a novel 66-kDa phosphoprotein. J Biol Chem 272:6621–6628. https://doi.org/10.1074/jbc.272.10.6621
Kang EH, Kim DJ, Lee EY et al (2009) Downregulation of heat shock protein 70 protects rheumatoid arthritis fibroblast-like synoviocytes from nitric oxide-induced apoptosis. Arthritis Res Ther 11:R130. https://doi.org/10.1186/ar2797
Kato M, Ospelt C, Gay RE et al (2014) Dual role of autophagy in stress-induced cell death in rheumatoid arthritis synovial fibroblasts. Arthritis Rheumatol 66:40–48. https://doi.org/10.1002/art.38190
Khan N, Mukhtar H (2013) Tea and health: studies in humans. Curr Pharm Des 19:6141–6147. https://doi.org/10.2174/1381612811319340008
Krock BL, Skuli N, Simon MC (2011) Hypoxia-induced angiogenesis: good and evil. Genes Cancer 2:1117–1133. https://doi.org/10.1177/1947601911423654
Leone M, Zhai D, Sareth S et al (2003) Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res 63:8118–8121
Liu W, Li J (2019) Theaflavin-3, 3′-digallate attenuates rheumatoid inflammation in mice through the nuclear factor-kappaB and MAPK Pathways. Arch Immunol Ther Exp 67:153–160. https://doi.org/10.1007/s00005-019-00536-7
Luo X, Zuo X, Zhou Y et al (2008) Extracellular heat shock protein 70 inhibits tumour necrosis factor-alpha induced proinflammatory mediator production in fibroblast-like synoviocytes. Arthritis Res Ther 10:R41. https://doi.org/10.1186/ar2399
MacDonald IJ, Liu SC, Su CM et al (2018) Implications of angiogenesis involvement in arthritis. Int J Mol Sci. https://doi.org/10.3390/ijms19072012
Nygaard G, Firestein GS (2020) Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat Rev Rheumatol 16:316–333. https://doi.org/10.1038/s41584-020-0413-5
Pan H, Li J, Rankin GO et al (2018) Synergistic effect of black tea polyphenol, theaflavin-3,3′-digallate with cisplatin against cisplatin resistant human ovarian cancer cells. J Funct Foods 46:1–11. https://doi.org/10.1016/j.jff.2018.04.037
Park YJ, Yoo SA, Kim WU (2014) Role of endoplasmic reticulum stress in rheumatoid arthritis pathogenesis. J Korean Med Sci 29:2–11. https://doi.org/10.3346/jkms.2014.29.1.2
Pincus T, Yazici Y, Sokka T et al (2003) Methotrexate as the “anchor drug” for the treatment of early rheumatoid arthritis. Clin Exp Rheumatol 21:S179–S185
Qu X, Tang Y, Hua S (2018) Immunological approaches towards cancer and inflammation: a cross talk. Front Immunol 9:563. https://doi.org/10.3389/fimmu.2018.00563
Quinonez-Flores CM, Gonzalez-Chavez SA, Pacheco-Tena C (2016) Hypoxia and its implications in rheumatoid arthritis. J Biomed Sci 23:62. https://doi.org/10.1186/s12929-016-0281-0
Runwal G, Stamatakou E, Siddiqi FH et al (2019) LC3-positive structures are prominent in autophagy-deficient cells. Sci Rep 9:10147. https://doi.org/10.1038/s41598-019-46657-z
Sakura T, Hayakawa F, Sugiura I et al (2018) High-dose methotrexate therapy significantly improved survival of adult acute lymphoblastic leukemia: a phase III study by JALSG. Leukemia 32:626–632. https://doi.org/10.1038/leu.2017.283
Shen Z, Chen Q, Jin T et al (2019) Theaflavin 3,3′-digallate reverses the downregulation of connexin 43 and autophagy induced by high glucose via AMPK activation in cardiomyocytes. J Cell Physiol 234:17999–18016. https://doi.org/10.1002/jcp.28432
Smith MD, Walker JG (2004) Apoptosis a relevant therapeutic target in rheumatoid arthritis? Rheumatology 43:405–407. https://doi.org/10.1093/rheumatology/keh084
Stebulis JA, Rossetti RG, Atez FJ, Zurier RB (2005) Fibroblast-like synovial cells derived from synovial fluid. J Rheumatol 32:301–306
Steinsson K, Weinstein A, Korn J, Abeles M (1982) Low dose methotrexate in rheumatoid arthritis. J Rheumatol 9:860–866
Tu Y, Kim E, Gao Y et al (2016) Theaflavin-3, 3′-digallate induces apoptosis and G2 cell cycle arrest through the Akt/MDM2/p53 pathway in cisplatin-resistant ovarian cancer A2780/CP70 cells. Int J Oncol 48:2657–2665. https://doi.org/10.3892/ijo.2016.3472
Vomero M, Barbati C, Colasanti T et al (2018) Autophagy and rheumatoid arthritis: current knowledges and future perspectives. Front Immunol 9:1577. https://doi.org/10.3389/fimmu.2018.01577
Wang M, Wey S, Zhang Y et al (2009) Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal 11:2307–2316. https://doi.org/10.1089/ARS.2009.2485
Wang W, Zhou H, Liu L (2018) Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur J Med Chem 158:502–516. https://doi.org/10.1016/j.ejmech.2018.09.027
Wu R, Zhang QH, Lu YJ et al (2015) Involvement of the IRE1α-XBP1 pathway and XBP1s-dependent transcriptional reprogramming in metabolic diseases. DNA Cell Biol 34:6–18. https://doi.org/10.1089/dna.2014.2552
Xu K, Cai YS, Lu SM et al (2015) Autophagy induction contributes to the resistance to methotrexate treatment in rheumatoid arthritis fibroblast-like synovial cells through high mobility group box chromosomal protein 1. Arthritis Res Ther 17:374. https://doi.org/10.1186/s13075-015-0892-y
Yang Y, Liu L, Naik I et al (2017) Transcription factor C/EBP homologous protein in health and diseases. Front Immunol 8:1612. https://doi.org/10.3389/fimmu.2017.01612
Yoo SA, You S, Yoon HJ et al (2012) A novel pathogenic role of the ER chaperone GRP78/BiP in rheumatoid arthritis. J Exp Med 209:871–886. https://doi.org/10.1084/jem.20111783
Yu MB, Firek A, Langridge WHR (2018) Predicting methotrexate resistance in rheumatoid arthritis patients. Inflammopharmacology 26:699–708. https://doi.org/10.1007/s10787-018-0459-z
Zeng L, Zampetaki A, Margariti A et al (2009) Sustained activation of XBP1 splicing leads to endothelial apoptosis and atherosclerosis development in response to disturbed flow. Proc Natl Acad Sci USA 106:8326–8331. https://doi.org/10.1073/pnas.0903197106
Zhu L, Wang H, Wu Y et al (2017) The autophagy level is increased in the synovial tissues of patients with active rheumatoid arthritis and is correlated with disease severity. Mediators Inflamm 2017:7623145. https://doi.org/10.1155/2017/7623145
Acknowledgements
The work was supported by National Tea Research Foundation, ICMR Senior Research Fellowship programme (ID:3/1/2/10/Ortho/2019-NCD-I) Govt. of India, DST-Inspire fellowship programme, Department of Science and Technology, Govt. of India, ICMR Research Associate Programme, Govt. of India.
Funding
This work was carried out with the support of the funding agency National Tea Research Foundation (NTRF 174/2015).
Author information
Authors and Affiliations
Contributions
SM, AS, SM, PSM, AG, PC and MC initiated the concept and designed the experiments. SM, AB, DB, SC, SC, AS performed the experiments. SN assisted in confocal experiments. SM, AS, SM, AB, PSM and AG wrote the manuscript. AC extracted the synovial fluid from patients and assisted in manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics approval
This study was approved by the Institutional Ethics Committee (No: Inst/IEC/24.02.2014) dated 24th February, 2014.
Consent to participate
This study was conducted using the biological samples collected from selected patients visited our OPD. For every patient, prior written consent was taken before enrolling him/her in the study.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Misra, S., Bagchi, A., Sarkar, A. et al. Methotrexate and theaflavin-3, 3′-digallate synergistically restore the balance between apoptosis and autophagy in synovial fibroblast of RA: an ex vivo approach with cultured human RA FLS. Inflammopharmacol 29, 1427–1442 (2021). https://doi.org/10.1007/s10787-021-00857-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-021-00857-0